| [1]Gomme PT, McCann KB, Bertolini J. Transferrin: structure, function and potential therapeutic actions. Drug Discov Today. 2005;10(4):267-273.[2]Daniels TR, Bernabeu E, Rodríguez JA, et al. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012;1820(3):291-317.[3]Soenen SJ, Vande Velde G, Ketkar-Atre A, et al. Magnetoliposomes as magnetic resonance imaging contrast agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(2):197-211.[4]陈鸿琪.蛋白质定量分析的进展[J].理化检验:化学分册. 2000, 36(7):33-36.[5]Kobayashi T, Ishida T, Okada Y, et al. Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. Int J Pharm. 2007;329(1-2):94-102. [6]Guo Y, Wang L, Lv P, et al. Transferrin-conjugated doxorubicin-loaded lipid-coated nanoparticles for the targeting and therapy of lung cancer. Oncol Lett. 2015;9(3):1065-1072.[7]Szwed M, Wrona D, Kania KD, et al. Doxorubicin-transferrin conjugate triggers pro-oxidative disorders in solid tumor cells. Toxicol In Vitro. 2016;31:60-71.[8]Salvatore A, Montis C, Berti D, et al. Multifunctional Magnetoliposomes for Sequential Controlled Release. ACS Nano. 2016;10(8):7749-7760. [9]Hodenius M, Würth C, Jayapaul J, et al. Fluorescent magnetoliposomes as a platform technology for functional and molecular MR and optical imaging. Contrast Media Mol Imaging. 2012;7(1):59-67. [10]Rodrigues AR, Gomes IT, Almeida BG, et al. Magnetic liposomes based on nickel ferrite nanoparticles for biomedical applications.Phys Chem Chem Phys. 2015;17(27):18011-18021. [11]Martins MB, Corvo ML, Marcelino P, et al. New long circulating magnetoliposomes as contrast agents for detection of ischemia-reperfusion injuries by MRI. Nanomedicine. 2014; 10(1):207-214.[12]Moore A, Josephson L, Bhorade RM, et al. Human transferrin receptor gene as a marker gene for MR imaging. Radiology. 2001;221(1):244-250.[13]Yang R, Tang Q, Miao F, et al. Inhibition of heat-shock protein 90 sensitizes liver cancer stem-like cells to magnetic hyperthermia and enhances anti-tumor effect on hepatocellular carcinoma-burdened nude mice. Int J Nanomedicine. 2015;10:7345-7358. [14]Ketkar-Atre A, Struys T, Dresselaers T, et al. In vivo hepatocyte MR imaging using lactose functionalized magnetoliposomes. Biomaterials. 2014;35(3):1015-1024.[15]Kozlowska D, Foran P, MacMahon P, et al. Molecular and magnetic resonance imaging: The value of immunoliposomes. Adv Drug Deliv Rev. 2009;61(15):1402-1411.[16]Manjappa AS, Chaudhari KR, Venkataraju MP, et al. Antibody derivatization and conjugation strategies: application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J Control Release. 2011;150(1):2-22.[17]Koshkaryev A, Sawant R, Deshpande M, et al. Immunoconjugates and long circulating systems: origins, current state of the art and future directions. Adv Drug Deliv Rev. 2013;65(1):24-35.[18]Miller AD. Lipid-based nanoparticles in cancer diagnosis and therapy. J Drug Deliv. 2013;2013:165981.[19]Vithanarachchi SM, Allen MJ. Strategies for Target-Specific Contrast Agents for Magnetic Resonance Imaging. Curr Mol Imaging. 2012;1(1):12-25.[20]Lei Y, Hamada Y, Li J, et al. Targeted tumor delivery and controlled release of neuronal drugs with ferritin nanoparticles to regulate pancreatic cancer progression. J Control Release. 2016;232:131-142.[21]Szwed M, Kania KD, Jozwiak Z. Assessment of pro-apoptotic activity of doxorubicin-transferrin conjugate in cells derived from human solid tumors. Int J Biochem Cell Biol. 2016;70: 57-67.[22]Zhang R, Li J, Li J, et al. Efficient In vitro labeling rabbit bone marrow-derived mesenchymal stem cells with SPIO and differentiating into neural-like cells. Mol Cells. 2014;37(9):650-655.[23] Gharib A, Faezizadeh Z, Mesbah-Namin SA, et al. Preparation, characterization and in vitro efficacy of magnetic nanoliposomes containing the artemisinin and transferrin. Daru. 2014;22:44.[24]Hauser AK, Wydra RJ, Stocke NA, et al. Magnetic nanoparticles and nanocomposites for remote controlled therapies. J Control Release. 2015;219:76-94.[25]Ferreira RV, Martins TM, Goes AM, et al. Thermosensitive gemcitabine-magnetoliposomes for combined hyperthermia and chemotherapy. Nanotechnology. 2016;27(8):085105. |
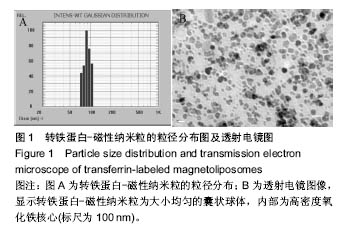
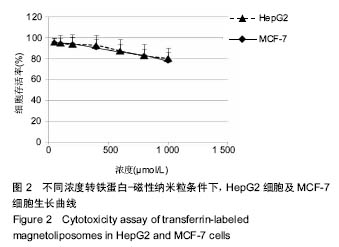
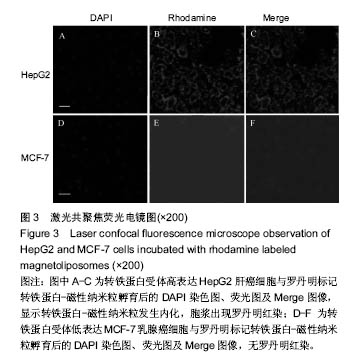
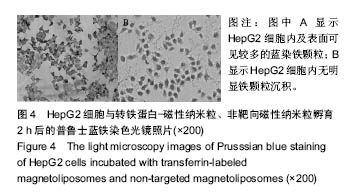
.jpg)

.jpg)