[1] ONOHUEAN H, AKIYODE AO, AKIYODE O, et al. Epidemiology of neurodegenerative diseases in the East African region: A meta-analysis. Front Neurol. 2022;13:1024004.
[2] BALANA AT, PRATT MR. Mechanistic roles for altered O-GlcNAcylation in neurodegenerative disorders. Biochem J. 2021;478(14):2733-2758.
[3] YIN R, WANG X, LI C, et al. Mass Spectrometry for O-GlcNAcylation. Front Chem. 2021;9:737093.
[4] CZAJEWSKI I, VAN AALTEN DMF. The role of O-GlcNAcylation in development. Development. 2023;150(6):dev201370.
[5] WU C, LI J, LU L, et al. OGT and OGA: Sweet guardians of the genome. J Biol Chem. 2024;300(4):107141.
[6] LAZARUS BD, LOVE DC, HANOVER JA. Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology. 2006;16(5):415-421.
[7] XIONG J, XU D. Mechanistic Insights into the Hydrolysis of O-GlcNAcylation Catalyzed by Human O-GlcNAcase. J Phys Chem B. 2020;124(42):9310-9322.
[8] ZEIDAN Q, HART GW. The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J Cell Sci. 2010; 123(Pt 1):13-22.
[9] MERGENTHALER P, LINDAUER U, DIENEL GA, et al. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36(10):587-597.
[10] MUHA V, FENCKOVA M, FERENBACH AT, et al. O-GlcNAcase contributes to cognitive function in Drosophila. J Biol Chem. 2020;295(26):8636-8646.
[11] REXACH JE, CLARK PM, MASON DE, et al. Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation. Nat Chem Biol. 2012;8(3):253-261.
[12] SHEN H, ZHAO X, CHEN J, et al. O-GlcNAc transferase Ogt regulates embryonic neuronal development through modulating Wnt/β-catenin signaling. Hum Mol Genet. 2021;31(1):57-68.
[13] TANWAR A, STANLEY P. Synergistic regulation of Notch signaling by different O-glycans promotes hematopoiesis. Front Immunol. 2023;14:1097332.
[14] DAS S, BAILEY SK, METGE BJ, et al. O-GlcNAcylation of GLI transcription factors in hyperglycemic conditions augments Hedgehog activity. Lab Invest. 2019;99(2):260-270.
[15] HAN S, KIM JN, PARK CH, et al. Modulation of synaptic transmission through O-GlcNAcylation. Mol Brain. 2024;17(1):1.
[16] LAGERLÖF O, HART GW, HUGANIR RL. O-GlcNAc transferase regulates excitatory synapse maturity. Proc Natl Acad Sci U S A. 2017; 114(7):1684-1689.
[17] CORTI E, DUARTE CB. The role of post-translational modifications in synaptic AMPA receptor activity. Biochem Soc Trans. 2023;51(1):315-330.
[18] YIN X, LI Y, FAN X, et al. SIRT1 deficiency increases O-GlcNAcylation of tau, mediating synaptic tauopathy. Mol Psychiatry. 2022;27(10):4323-4334.
[19] KNOPMAN DS, AMIEVA H, PETERSEN RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33.
[20] LAO K, ZHANG R, LUAN J, et al. Therapeutic Strategies Targeting Amyloid-β Receptors and Transporters in Alzheimer’s Disease. J Alzheimers Dis. 2021;79(4): 1429-1442.
[21] HUANG CW, RUST NC, WU HF, et al. Low glucose induced Alzheimer’s disease-like biochemical changes in human induced pluripotent stem cell-derived neurons is due to dysregulated O-GlcNAcylation. Alzheimers Dement. 2023;19(11):4872-4885.
[22] AHMAD W. Glucose enrichment impair neurotransmission and induce Aβ oligomerization that cannot be reversed by manipulating O-β-GlcNAcylation in the C. elegans model of Alzheimer’s disease. J Nutr Biochem. 2022;108:109100.
[23] NEHRA G, PROMSAN S, YUBOLPHAN R, et al. Cognitive decline, Aβ pathology, and blood-brain barrier function in aged 5xFAD mice. Fluids Barriers CNS. 2024; 21(1):29.
[24] LI S, QU L, WANG X, et al. Novel insights into RIPK1 as a promising target for future Alzheimer’s disease treatment. Pharmacol Ther. 2022; 231:107979.
[25] DONG X, SHU L, ZHANG J, et al. Ogt-mediated O-GlcNAcylation inhibits astrocytes activation through modulating NF-κB signaling pathway.J Neuroinflammation.2023;20(1):146.
[26] RANI L, MALLAJOSYULA SS. Phosphorylation-Induced Structural Reorganization in Tau-Paired Helical Filaments. ACS Chem Neurosci. 2021;12(9):1621-1631.
[27] OTERO-GARCIA M, MAHAJANI SU, WAKHLOO D, et al. Molecular signatures underlying neurofibrillary tangle susceptibility in Alzheimer’s disease. Neuron. 2022;110(18):2929-2948.e8.
[28] PAN D, GU JH, ZHANG J, et al. Thiamme2-G, a Novel O-GlcNAcase Inhibitor, Reduces Tau Hyperphosphorylation and Rescues Cognitive Impairment in Mice. J Alzheimers Dis. 2021;81(1):273-286.
[29] ROSTGAARD N, JUL PH, GARMER M, et al. Increasing O-GlcNAcylation Attenuates tau Hyperphosphorylation and Behavioral Impairment in rTg4510 Tauopathy Mice. J Integr Neurosci. 2023;22(5):135.
[30] CANTRELLE FX, LOYENS A, TRIVELLI X, et al. Phosphorylation and O-GlcNAcylation of the PHF-1 Epitope of Tau Protein Induce Local Conformational Changes of the C-Terminus and Modulate Tau Self-Assembly Into Fibrillar Aggregates. Front Mol Neurosci. 2021;14:661368.
[31] YUZWA SA, CHEUNG AH, OKON M, et al. O-GlcNAc modification of tau directly inhibits its aggregation without perturbing the conformational properties of tau monomers. J Mol Biol. 2014;426(8):1736-1752.
[32] LU S, YIN X, WANG J, et al. SIRT1 regulates O-GlcNAcylation of tau through OGT. Aging (Albany NY). 2020;12(8):7042-7055.
[33] LO RY. Epidemiology of atypical parkinsonian syndromes. Tzu Chi Med J. 2021; 34(2):169-181.
[34] 齐雪,李家慧,朱远峰,等.α-突触核蛋白的异常修饰及在帕金森病中的作用机制[J].中国组织工程研究,2024,28(8):1301-1306.
[35] TAVASSOLY O, YUE J, VOCADLO DJ. Pharmacological inhibition and knockdown of O-GlcNAcase reduces cellular internalization of α-synuclein preformed fibrils. FEBS J. 2021;288(2):452-470.
[36] LEE BE, KIM HY, KIM HJ, et al. O-GlcNAcylation regulates dopamine neuron function, survival and degeneration in Parkinson disease. Brain. 2020;143(12): 3699-3716.
[37] RADEMACHER K, NAKAMURA K. Role of dopamine neuron activity in Parkinson’s disease pathophysiology. Exp Neurol. 2024;373:114645.
[38] LEE BE, SUH PG, KIM JI. O-GlcNAcylation in health and neurodegenerative diseases. Exp Mol Med. 2021;53(11):1674-1682.
[39] MEJZINI R, FLYNN LL, PITOUT IL, et al. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front Neurosci. 2019;13:1310.
[40] LOBSIGER CS, GARCIA ML, WARD CM, et al. Altered axonal architecture by removal of the heavily phosphorylated neurofilament tail domains strongly slows superoxide dismutase 1 mutant-mediated ALS. Proc Natl Acad Sci U S A. 2005;102(29):10351-10356.
[41] ZHAO MJ, YAO X, WEI P, et al. O-GlcNAcylation of TDP-43 suppresses proteinopathies and promotes TDP-43’s mRNA splicing activity. EMBO Rep. 2021;22(6):e51649.
[42] HSIEH YL, SU FY, TSAI LK, et al. NPGPx-Mediated Adaptation to Oxidative Stress Protects Motor Neurons from Degeneration in Aging by Directly Modulating O-GlcNAcase. Cell Rep. 2019;29(8):2134-2143.e7.
[43] MCCOLGAN P, TABRIZI SJ. Huntington’s disease: a clinical review. Eur J Neurol. 2018;25(1):24-34.
[44] HO LW, BROWN R, MAXWELL M, et al. Wild type Huntingtin reduces the cellular toxicity of mutant Huntingtin in mammalian cell models of Huntington’s disease. J Med Genet. 2001;38(7):450-452.
[45] KUMAR A, SINGH PK, PARIHAR R, et al. Decreased O-linked GlcNAcylation protects from cytotoxicity mediated by huntingtin exon1 protein fragment. J Biol Chem. 2014;289(19):13543-13553.
[46] WANG P, LAZARUS BD, FORSYTHE ME, et al. O-GlcNAc cycling mutants modulate proteotoxicity in Caenorhabditis elegans models of human neurodegenerative diseases. Proc Natl Acad Sci U S A. 2012;109(43):17669-17674.
[47] GRIMA JC, DAIGLE JG, ARBEZ N, et al. Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron. 2017;94(1):93-107.e6.
[48] ZHU Y, LIU TW, MADDEN Z, et al. Post-translational O-GlcNAcylation is essential for nuclear pore integrity and maintenance of the pore selectivity filter. J Mol Cell Biol. 2016;8(1):2-16.
[49] LOI EM, TOMAŠIČ T, BALSOLLIER C, et al. Discovery of a New Drug-like Series of OGT Inhibitors by Virtual Screening. Molecules. 2022;27(6):1996.
[50] HALTIWANGER RS, GROVE K, PHILIPSBERG GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998; 273(6):3611-3617.
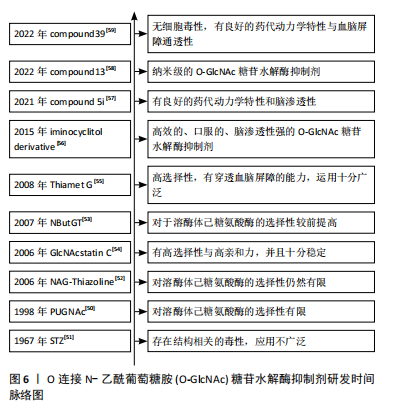
[51] HERR RR, JAHNKE JK, ARGOUDELIS AD. The structure of streptozotocin. J Am Chem Soc. 1967;89(18):4808-4809.
[52] CETINBAŞ N, MACAULEY MS, STUBBS KA, et al. Identification of Asp174 and Asp175 as the key catalytic residues of human O-GlcNAcase by functional analysis of site-directed mutants. Biochemistry. 2006;45(11):3835-3844.
[53] WHITWORTH GE, MACAULEY MS, STUBBS KA, et al. Analysis of PUGNAc and NAG-thiazoline as transition state analogues for human O-GlcNAcase: mechanistic and structural insights into inhibitor selectivity and transition state poise. J Am Chem Soc. 2007;129(3):635-644.
[54] DORFMUELLER HC, BORODKIN VS, SCHIMPL M, et al. GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-glcNAcylation levels. J Am Chem Soc. 2006;128(51):16484-16485.
[55] YUZWA SA, MACAULEY MS, HEINONEN JE, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4(8):483-490.
[56] BERGERON-BRLEK M, GOODWIN-TINDALL J, CEKIC N, et al. A Convenient Approach to Stereoisomeric Iminocyclitols: Generation of Potent Brain-Permeable OGA Inhibitors. Angew Chem Int Ed Engl. 2015;54(51):15429-15433.
[57] TAWADA M, FUSHIMI M, MASUDA K, et al. Discovery of a Novel and Brain-Penetrant O-GlcNAcase Inhibitor via Virtual Screening, Structure-Based Analysis, and Rational Lead Optimization. J Med Chem. 2021;64(2):1103-1115.
[58] WEBER P, MÉSZÁROS Z, JAGEČIĆ D, et al. Diaminocyclopentane-derived O-GlcNAcase inhibitors for combating tau hyperphosphorylation in Alzheimer’s disease. Chem Commun (Camb). 2022;58(63):8838-8841.
[59] LI X, HAN J, BUJARANIPALLI S, et al. Structure-based discovery and development of novel O-GlcNAcase inhibitors for the treatment of Alzheimer’s disease. Eur J Med Chem. 2022;238:114444.
[60] SELNICK HG, HESS JF, TANG C, et al. Discovery of MK-8719, a Potent O-GlcNAcase Inhibitor as a Potential Treatment for Tauopathies. J Med Chem. 2019;62(22): 10062-10097.
[61] WANG X, LI W, MARCUS J, et al. MK-8719, a Novel and Selective O-GlcNAcase Inhibitor That Reduces the Formation of Pathological Tau and Ameliorates Neurodegeneration in a Mouse Model of Tauopathy. J Pharmacol Exp Ther. 2020;374(2):252-263.
[62] PERMANNE B, SAND A, OUSSON S, et al. O-GlcNAcase Inhibitor ASN90 is a Multimodal Drug Candidate for Tau and α-Synuclein Proteinopathies. ACS Chem Neurosci. 2022;13(8):1296-1314.
[63] DU P, ZHANG X, LIAN X, et al. O-GlcNAcylation and Its Roles in Neurodegenerative Diseases. J Alzheimers Dis. 2024;97(3):1051-1068.
[64] PRATT MR, VOCADLO DJ. Understanding and exploiting the roles of O-GlcNAc in neurodegenerative diseases. J Biol Chem. 2023;299(12): 105411.
[65] MA X, LI H, HE Y, et al. The emerging link between O-GlcNAcylation and neurological disorders. Cell Mol Life Sci. 2017;74(20):3667-3686.
[66] ALTEEN MG, TAN HY, VOCADLO DJ. Monitoring and modulating O-GlcNAcylation: assays and inhibitors of O-GlcNAc processing enzymes. Curr Opin Struct Biol. 2021;68:157-165. |