中国组织工程研究 ›› 2020, Vol. 24 ›› Issue (17): 2742-2751.doi: 10.3969/j.issn.2095-4344.2612
• 组织构建临床实践 clinical practice in tissue construction • 上一篇 下一篇
基于ClinicalTrials.gov注册平台分析的组织工程临床试验注册的现状
卢 岩,张 婷,欧阳昭连
- 中国医学科学院医学信息研究所,北京市 100020
Current status of clinical trial registrations in tissue engineering based on ClinicalTrials.gov
Lu Yan, Zhang Ting, Ouyang Zhaolian
- Institute of Medical Information, CAMS & PUMC, Beijing 100020, China
摘要:
文题释义:
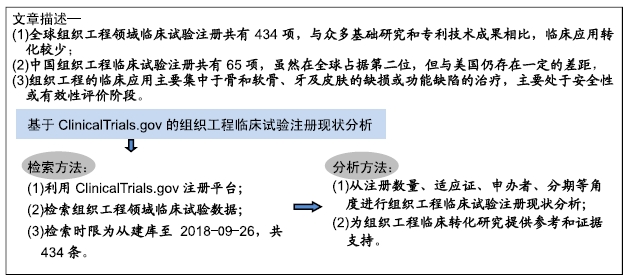
ClinicalTrials.gov注册平台:ClinicalTrials.gov注册平台现由美国国立卫生研究院(National Institutes of Health,NIH)国家医学图书馆(National Library of Medicine,NLM)维护,是国际上使用最普遍的临床试验注册平台,涵盖了美国50个州和另外208个国家/地区开展的多个领域的近30万项临床试验;此外,在该平台进行临床试验注册符合国际医学期刊编辑委员会(International Committee of Medical Journal Editors,ICMJE)对相关论文发表的要求。
对组织工程临床转化研究的意义:基于ClinicalTrials.gov注册平台中的临床试验注册数据,通过对组织工程领域临床试验的注册数量、适应证、申办者、分期等角度进行统计分析,全面把握该领域的临床试验注册现状,以期为组织工程临床转化研究提供参考和证据支持。
背景:组织器官市场供求不平衡,积极开展组织工程基础研究并加快临床转化至关重要。
目的:对组织工程临床试验注册现状进行整体分析,为组织工程临床转化研究提供参考和证据支持。
方法:通过ClinicalTrials.gov注册平台采集数据,利用文献计量法和数理统计法,从注册数量、适应证、申办者、分期等角度对组织工程临床试验注册现状进行分析。
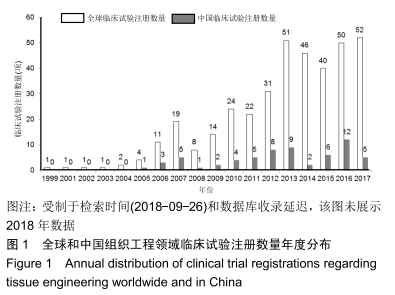
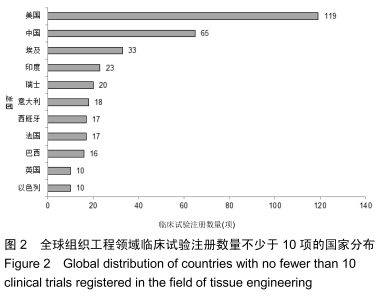
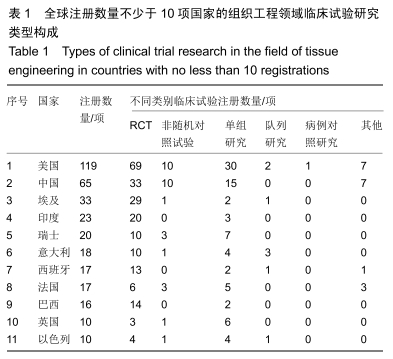
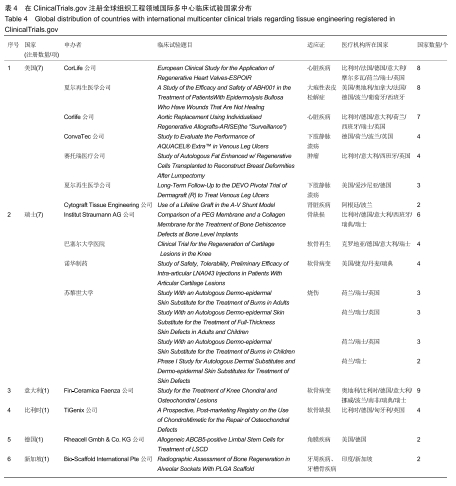
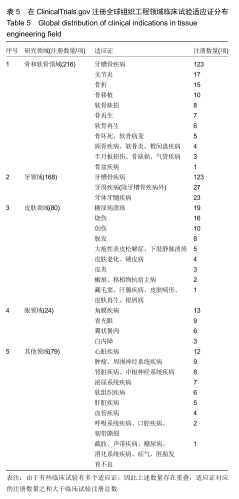
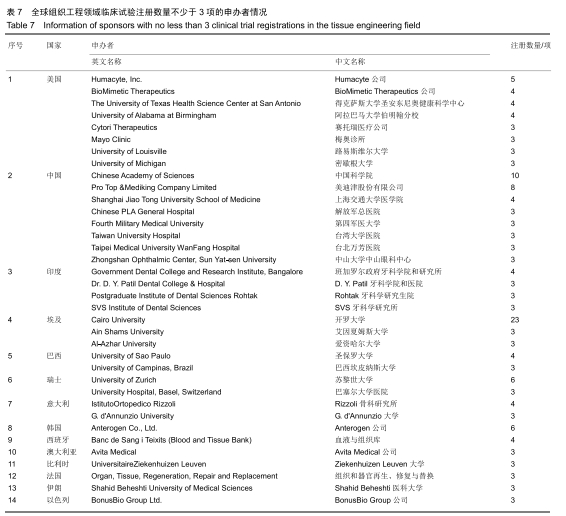
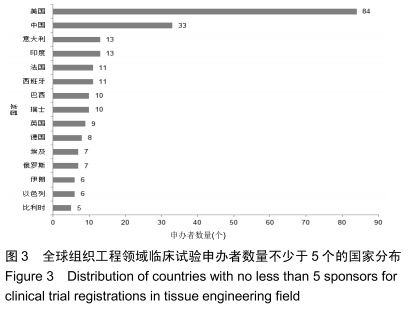
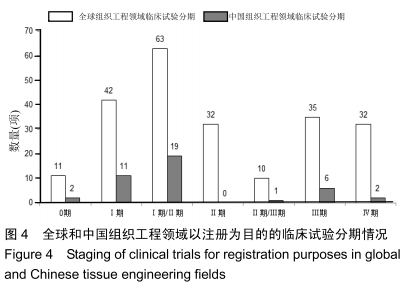
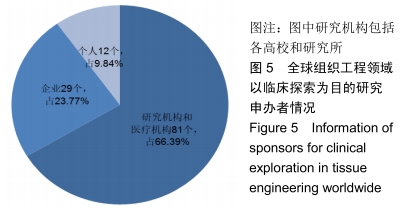
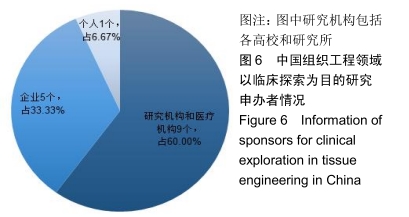
结果与结论:①全球组织工程领域临床试验434项,中国65项;全球和中国均是超过一半为随机对照试验,全球18项国际多中心临床试验中未见中国机构参与;全球和中国分别涵盖适应证57种、37种,主要集中于骨和软骨、牙、皮肤等领域;全球申办者275个,中国33个;②全球和中国均是Ⅰ期/Ⅱ期临床试验注册数量最多;③通过对组织工程临床试验注册现状的分析发现,组织工程领域的临床应用转化较少,中国虽然注册数量在全球占据第二位,但与美国仍存在一定差距;已经积累了一定数量的高证据级别的临床试验,但缺乏高潜在国际市场价值的产品;研究机构和医疗机构对临床转化研究更为重视,临床应用主要集中于骨和软骨、牙及皮肤的缺损或功能缺陷的治疗,主要处于安全性或有效性评价阶段。
ORCID: 0000-0002-7227-732X(卢岩)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号: