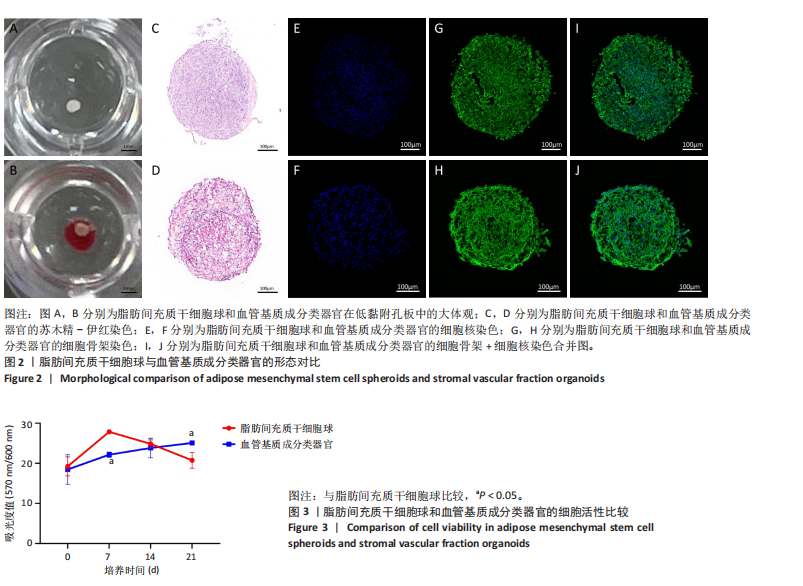
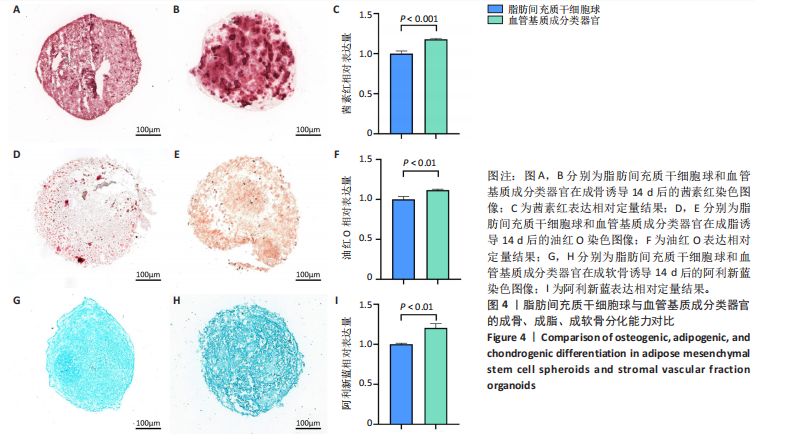
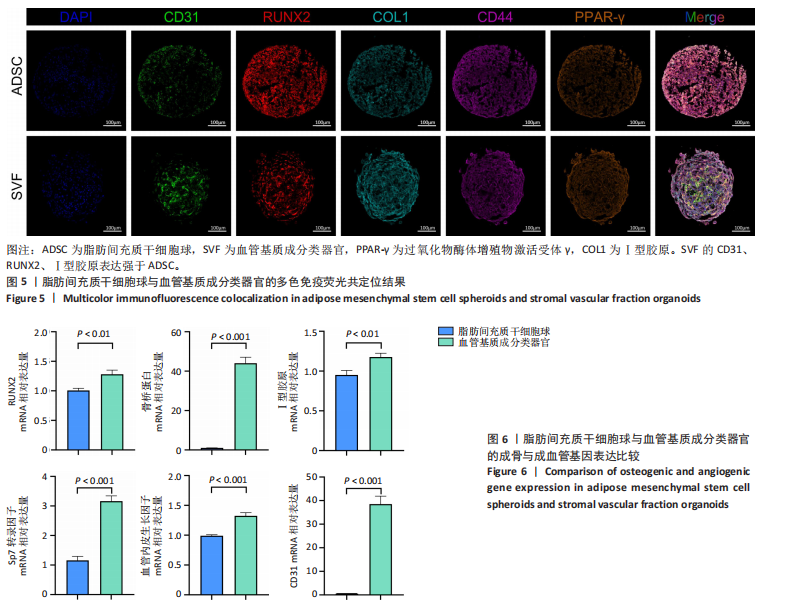
[1] SIMIAN M, BISSELL MJ. Organoids: A historical perspective of thinking in three dimensions. J Cell Biol. 2017;216(1):31-40.
[2] CHEN CS, MRKSICH M, HUANG S, et al. Geometric control of cell life and death. Science. 1997;276(5317):1425-1428.
[3] SHAO Y, SANG J, FU J. On human pluripotent stem cell control: The rise of 3D bioengineering and mechanobiology. Biomaterials. 2015;52:26-43.
[4] VEGA SL, KWON MY, SONG KH, et al. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat Commun. 2018;9(1):614.
[5] SASAI Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12(5):520-530.
[6] WANG J, WU Y, LI G, et al. Engineering Large-Scale Self-Mineralizing Bone Organoids with Bone Matrix-Inspired Hydroxyapatite Hybrid Bioinks. Adv Mater. 2024;36(30):e2309875.
[7] GROSSO A, BURGER MG, LUNGER A, et al. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front Bioeng Biotechnol. 2017;5:68.
[8] LEE J, LEE S, AHMAD T, et al. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255:120192.
[9] THERY A, BLÉRY P, MALARD O, et al. Role of the stromal vascular fraction from adipose tissue in association with a phosphocalcic scaffold in bone regeneration in an irradiated area. J Craniomaxillofac Surg. 2015; 43(7):1169-1176.
[10] STAMNITZ S, KLIMCZAK A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells. 2021;10(8):1925.
[11] ROHRINGER S, HOFBAUER P, SCHNEIDER KH, et al. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis. 2014; 17(4):921-933.
[12] HOLNTHONER W, HOHENEGGER K, HUSA AM, et al. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med. 2015;9(2):127-136.
[13] YAN Y, CHEN H, ZHANG H, et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials. 2019;190-191:97-110.
[14] KWON HM, HUR SM, PARK KY, et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol. 2014;63(1):19-28.
[15] WANG T, LI T, NIU X, et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol Direct. 2023;18(1):6.
[16] HEPLER C, VISHVANATH L, GUPTA RK. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev. 2017;31(2):127-140.
[17] HAN S, SUN HM, HWANG KC, et al. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit Rev Eukaryot Gene Expr. 2015;25(2):145-152.
[18] FENNEMA EM, TCHANG LAH, YUAN H, et al. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med. 2018;12(1): e150-e158.
[19] SINGH S, NYBERG EL, O’SULLIVAN AN, et al. Point-of-care treatment of geometrically complex midfacial critical-sized bone defects with 3D-Printed scaffolds and autologous stromal vascular fraction. Biomaterials. 2022;282:121392.
[20] FILIPPI M, DASEN B, GUERRERO J, et al. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials. 2019;223:119468.
[21] EPPLE C, HAUMER A, ISMAIL T, et al. Prefabrication of a large pedicled bone graft by engineering the germ for de novo vascularization and osteoinduction. Biomaterials. 2019;192:118-127.
[22] WITTMANN K, DIETL S, LUDWIG N, et al. Engineering vascularized adipose tissue using the stromal-vascular fraction and fibrin hydrogels. Tissue Eng Part A. 2015;21(7-8):1343-1353.
[23] VERMETTE M, TROTTIER V, MÉNARD V, et al. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials. 2007;28(18):2850-2860.
[24] LOUIS F, SOWA Y, IRIE S, et al. Injectable Prevascularized Mature Adipose Tissues (iPAT) to Achieve Long-Term Survival in Soft Tissue Regeneration. Adv Healthc Mater. 2022;11(23):e2201440.
[25] ESCUDERO M, VAYSSE L, EKE G, et al. Scalable Generation of Pre-Vascularized and Functional Human Beige Adipose Organoids. Adv Sci (Weinh). 2023;10(31):e2301499.
[26] ROBLEDO F, GONZÁLEZ-HODAR L, TAPIA P, et al. Spheroids derived from the stromal vascular fraction of adipose tissue self-organize in complex adipose organoids and secrete leptin. Stem Cell Res Ther. 2023;14(1):70.
[27] STROBEL HA, GERTON T, HOYING JB. Vascularized adipocyte organoid model using isolated human microvessel fragments. Biofabrication. 2021;13(3). doi: 10.1088/1758-5090/abe187.
[28] LANCASTER MA, KNOBLICH JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014; 345(6194):1247125.
[29] BROUTIER L, ANDERSSON-ROLF A, HINDLEY CJ, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11(9):1724-1743.
[30] SATO T, VRIES RG, SNIPPERT HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009; 459(7244):262-265.
[31] MILLS RJ, PARKER BL, QUAIFE-RYAN GA, et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell. 2019;24(6):895-907.e6.
[32] MUN SJ, RYU JS, LEE MO, et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71(5): 970-985.
[33] LANCASTER MA, RENNER M, MARTIN CA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013; 501(7467):373-379.
[34] DUTTA D, HEO I, CLEVERS H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol Med. 2017;23(5):393-410.
[35] RANGA A, GJOREVSKI N, LUTOLF MP. Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 2014;69-70:19-28.
[36] KALUTHANTRIGE DON F, HUCH M. Organoids, Where We Stand and Where We Go. Trends Mol Med. 2021;27(5):416-418.
[37] YAGHOUBI Y, MOVASSAGHPOUR A, ZAMANI M, et al. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733.
[38] PAMPALONI F, REYNAUD EG, STELZER EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007; 8(10):839-845.
[39] ZURINA IM, GORKUN AA, DZHUSSOEVA EV, et al. Human Melanocyte-Derived Spheroids: A Precise Test System for Drug Screening and a Multicellular Unit for Tissue Engineering. Front Bioeng Biotechnol. 2020;8:540.
[40] LIU X, LI L, GAIHRE B, et al. Scaffold-Free Spheroids with Two-Dimensional Heteronano-Layers (2DHNL) Enabling Stem Cell and Osteogenic Factor Codelivery for Bone Repair. ACS Nano. 2022;16(2):2741-2755.
[41] MULLER S, ADER I, CREFF J, et al. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep. 2019;9(1):7250.
[42] ROATO I, BELISARIO DC, COMPAGNO M, et al. Adipose-Derived Stromal Vascular Fraction/Xenohybrid Bone Scaffold: An Alternative Source for Bone Regeneration. Stem Cells Int. 2018;2018:4126379.
[43] CHAABAN M, MOYA A, GARCÍA-GARCÍA A, et al. Harnessing human adipose-derived stromal cell chondrogenesis in vitro for enhanced endochondral ossification. Biomaterials. 2023;303:122387.
|