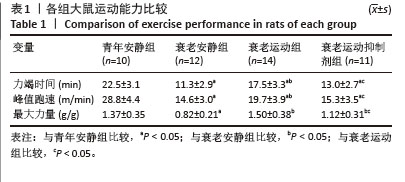
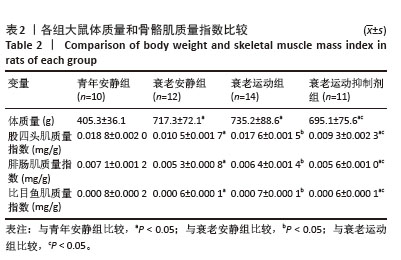
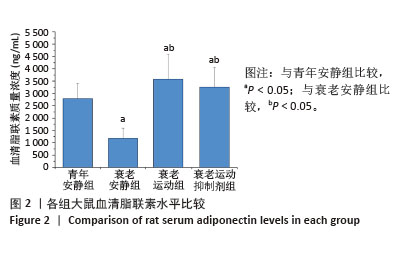
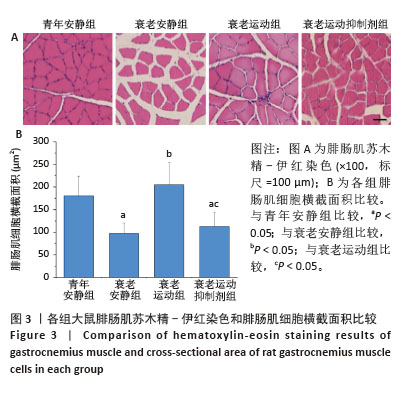
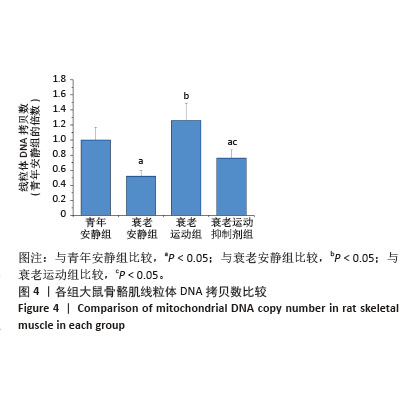
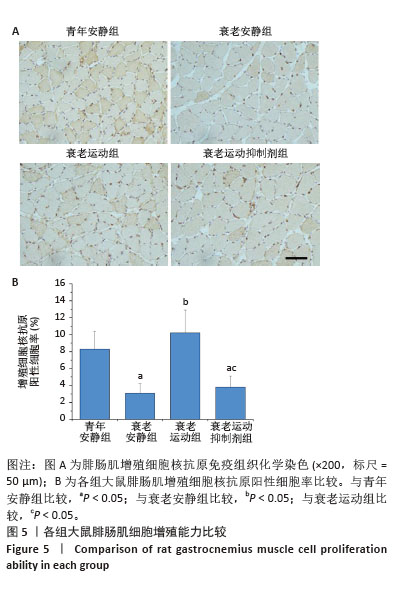
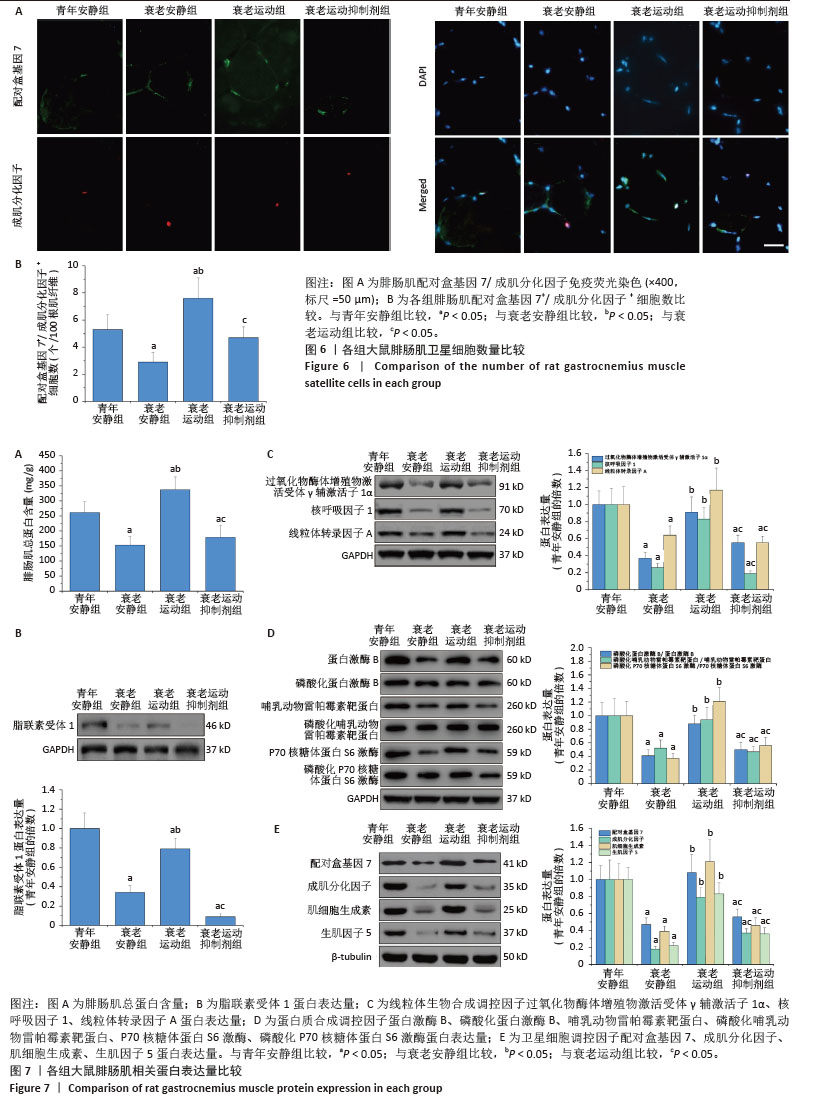
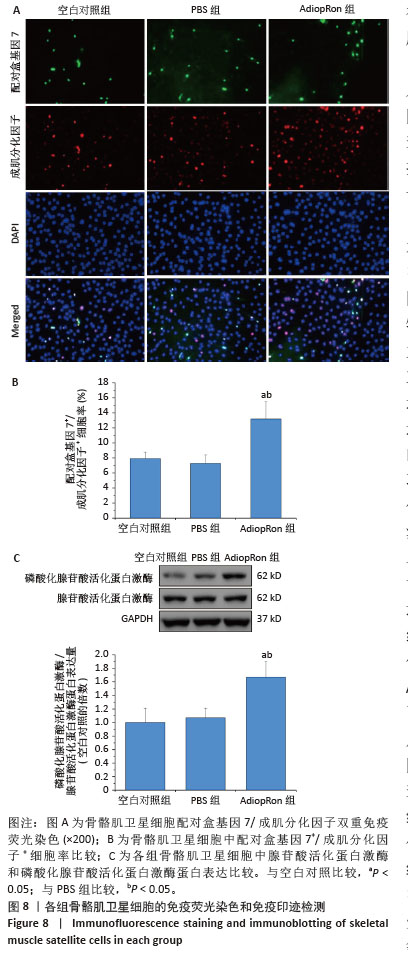
[1] LIM JY, FRONTERA WR. Single skeletal muscle fiber mechanical properties: A muscle quality biomarker of human aging. Eur J Appl Physiol. 2022;122(6):1383-1395.
[2] NARUSE M, TRAPPE S, TRAPPE TA. Human skeletal muscle-specific atrophy with aging: a comprehensive review. J Appl Physiol (1985). 2023;134(4):900-914.
[3] KANG YK, MIN B, EOM J, et al. Different phases of aging in mouse old skeletal muscle. Aging (Albany NY). 2022;14(1):143-160.
[4] MARZUCA-NASSR GN, SANMARTíN-CALíSTO Y, GUERRA-VEGA P, et al. Skeletal muscle aging atrophy: assessment and exercise-based treatment. Adv Exp Med Biol. 2020;1260:123-158.
[5] GUILHOT C, CATENACCI M, LOFARO S, et al. The satellite cell in skeletal muscle: A story of heterogeneity. Curr Top Dev Biol. 2024;158: 15-51.
[6] BACHMAN JF, CHAKKALAKAL JV. Satellite cells in the growth and maintenance of muscle. Curr Top Dev Biol. 2024;158:1-14.
[7] SOUSA-VICTOR P, GARCÍA-PRAT L, MUÑOZ-CÁNOVES P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol. 2022;23(3):204-226.
[8] KIM YA, KIM YS, OH SL, et al. Autophagic response to exercise training in skeletal muscle with age. J Physiol Biochem. 2013;69(4): 697-705.
[9] BETIK AC, THOMAS MM, WRIGHT KJ, et al. Exercise training from late middle age until senescence does not attenuate the declines in skeletal muscle aerobic function. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R744-755.
[10] CURRIER BS, MCLEOD JC, BANFIELD L, et al. Resistance training prescription for muscle strength and hypertrophy in healthy adults: A systematic review and bayesian network meta-analysis. Br J Sports Med. 2023;57(18):1211-1220.
[11] 付常喜,何瑞波,马刚,等.不同运动模式影响心肌梗死致心力衰竭大鼠骨骼肌重塑的机制[J].中国组织工程研究,2025,29(2): 221-230.
[12] LI Y, ONODERA T, SCHERER PE. Adiponectin. Trends Endocrinol Metab. 2024;35(7):674-675.
[13] LI P, ZHANG S, SONG H, et al. Naringin promotes skeletal muscle fiber remodeling by the Adipor1-APPL1-AMPK signaling pathway. J Agric Food Chem. 2021;69(40):11890-11899.
[14] LI N, ZHAO S, ZHANG Z, et al. Adiponectin preserves metabolic fitness during aging. Elife. 2021;10:e65108.
[15] CHEN MB, MCAINCH AJ, MACAULAY SL, et al. Impaired activation of amp-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90(6):3665-3672.
[16] MURASE T, HARAMIZU S, OTA N, et al. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. 2009;10(4):423-434.
[17] LAKHDAR N, DENGUEZLI M, ZAOUALI M, et al. Six months training alone or combined with diet alters HOMA-AD, HOMA-IR and plasma and adipose tissue adiponectin in obese women. Neuro Endocrinol Lett. 2014;35(5):373-379.
[18] 朱政,付常喜,马文超,等.有氧运动调控自发性高血压模型大鼠心脏重塑的机制[J].中国组织工程研究,2022,26(14):2231-2237.
[19] DE SOUSA NETO IV, DURIGAN J, CARREIRO DE FARIAS JUNIOR G, et al. Resistance training modulates the matrix metalloproteinase-2 activity in different trabecular bones in aged rats. Clin Interv Aging. 2021;16:71-81.
[20] IKEDO A, KIDO K, ATO S, et al. The effects of resistance training on bone mineral density and bone quality in type 2 diabetic rats. Physiol Rep. 2019;7(6):e14046.
[21] TANG L, ZHAO T, KANG Y, et al. MSTN is an important myokine for weight-bearing training to attenuate bone loss in ovariectomized rats. J Physiol Biochem. 2022;78(1):61-72.
[22] KELTY TJ, MAO X, KERR NR, et al. Resistance-exercise training attenuates LPS-induced astrocyte remodeling and neuroinflammatory cytokine expression in female wistar rats. J Appl Physiol (1985). 2022; 132(2):317-326.
[23] STARON RS, HAGERMAN FC, HIKIDA RS, et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48(5):623-629.
[24] TANG G, DU Y, GUAN H, et al. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br J Pharmacol. 2022; 179(1):159-178.
[25] BASU U, BOSTWICK AM, DAS K, et al. Structure, mechanism, and regulation of mitochondrial DNA transcription initiation. J Biol Chem. 2020;295(52):18406-18425.
[26] HALLING JF, PILEGAARD H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl Physiol Nutr Metab. 2020;45(9):927-936.
[27] LIM C, NUNES EA, CURRIER BS, et al. An evidence-based narrative review of mechanisms of resistance exercise-induced human skeletal muscle hypertrophy. Med Sci Sports Exerc. 2022;54(9):1546-1559.
[28] HSU WB, LIN SJ, HUNG JS, et al. Effect of resistance training on satellite cells in old mice - a transcriptome study : Implications for sarcopenia. Bone Joint Res. 2022;11(2):121-133.
[29] GALLAGHER H, HENDRICKSE PW, PEREIRA MG, et al. Skeletal muscle atrophy, regeneration, and dysfunction in heart failure: Impact of exercise training. J Sport Health Sci. 2023;12(5):557-567.
[30] FUKADA SI, NAKAMURA A. Exercise/resistance training and muscle stem cells. Endocrinol Metab (Seoul). 2021;36(4):737-744.
[31] HICKS MR, SALEH KK, CLOCK B, et al. Regenerating human skeletal muscle forms an emerging niche in vivo to support PAX7 cells. Nat Cell Biol. 2023;25(12):1758-1773.
[32] SHEPHERD DW, NORRIS JM, SIMPSON BS, et al. Effects of photobiomodulation therapy on regulation of myogenic regulatory factor mRNA expression in vivo: A systematic review. J Biophotonics. 2022;15(2):e202100219.
[33] KONG L, FANG Y, DU M, et al. Gαi2 regulates the adult myogenesis of masticatory muscle satellite cells. J Cell Mol Med. 2023;27(9): 1239-1249.
[34] CARDONE N, TAGLIETTI V, BARATTO S, et al. Myopathologic trajectory in duchenne muscular dystrophy (DMD) reveals lack of regeneration due to senescence in satellite cells. Acta Neuropathol Commun. 2023; 11(1):e167.
[35] CHERIKI M, HABIBIAN M, MOOSAVI SJ. Curcumin attenuates brain aging by reducing apoptosis and oxidative stress. Metab Brain Dis. 2024;39(5):833-840.
[36] SONG ZW, JIN CL, YE M, et al. Lysine inhibits apoptosis in satellite cells to govern skeletal muscle growth via the JAK2-STAT3 pathway. Food Funct. 2020;11(5):3941-3951.
[37] CHEN X, XIANG L, JIA G, et al. Leucine regulates slow-twitch muscle fibers expression and mitochondrial function by Sirt1/AMPK signaling in porcine skeletal muscle satellite cells. Anim Sci J. 2019;90(2):255-263.
[38] FU X, ZHU MJ, DODSON MV, et al. AMP-activated protein kinase stimulates Warburg-like glycolysis and activation of satellite cells during muscle regeneration. J Biol Chem. 2015;290(44): 26445-26456.
[39] SU R, WANG B, ZHANG M, et al. Effects of energy supplements on the differentiation of skeletal muscle satellite cells. Food Sci Nutr. 2021;9(1):357-366.
[40] VILCHINSKAYA NA, ROZHKOV SV, TURTIKOVA OV, et al. AMPK phosphorylation impacts apoptosis in differentiating myoblasts isolated from atrophied rat soleus muscle. Cells. 2023;12(6):e920. |