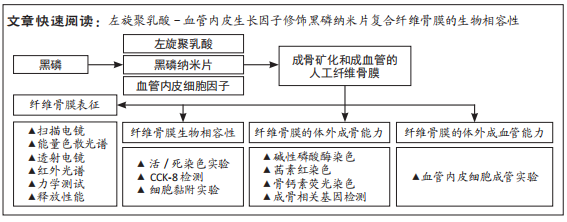
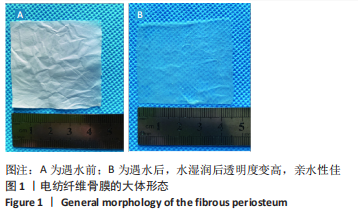
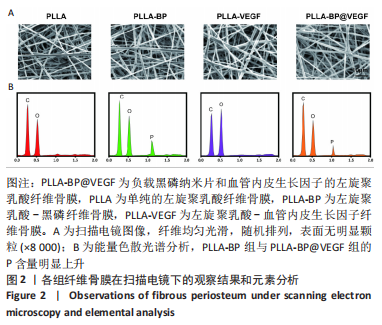
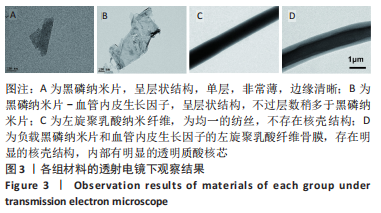
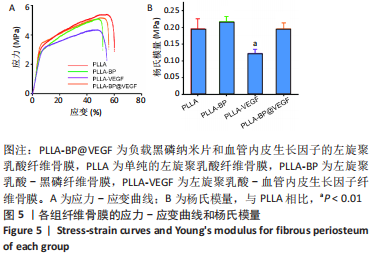
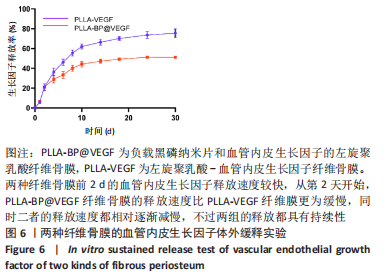
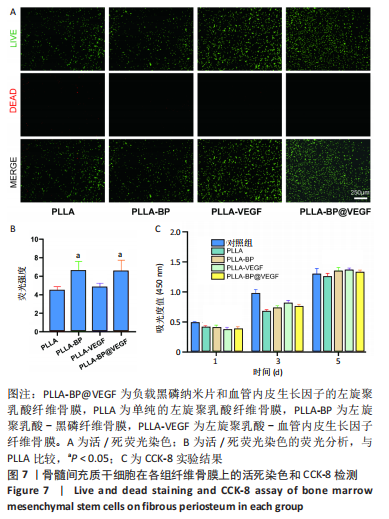
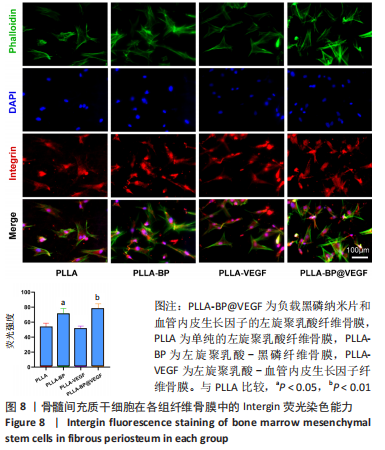
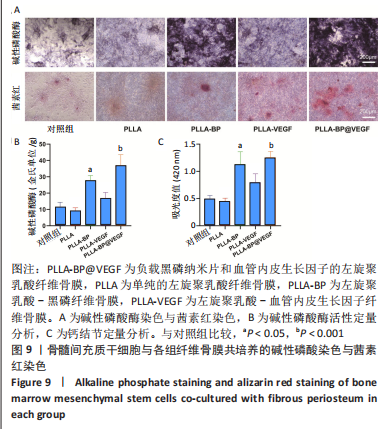
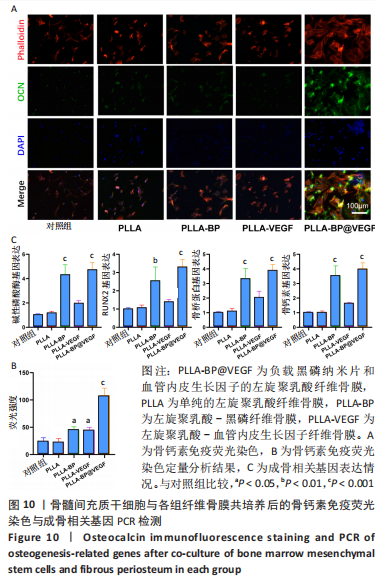
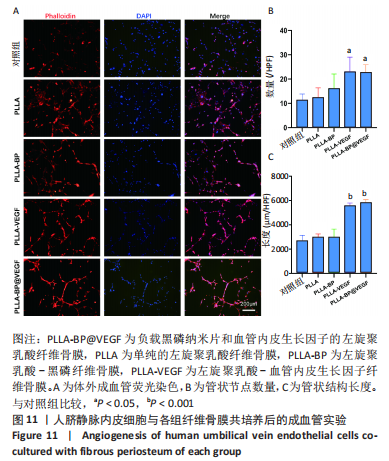
[1] AREALIS G, NIKOLAOU VS. Bone printing: new frontiers in the treatment of bone defects. Injury. 2015;46:S20-S22.
[2] VERRIER S, ALINI M, ALSBERG E, et al. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Eur Cell Mater. 2016;32:87-110.
[3] ALONZO M, PRIMO FA, KUMAR SA, et al. Bone tissue engineering techniques, advances and scaffolds for treatment of bone defects. Curr Opin Biomed Eng. 2021;17:100248.
[4] TANG D, TARE RS, YANG LY, et al. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363-382.
[5] BADYLAK SF, NEREM RM. Progress in tissue engineering and regenerative medicine. Proceed Nat Acad Sci. 2010;107(8):3285-3286.
[6] YANG G, LIU H, CUI Y, et al. Bioinspired membrane provides periosteum-mimetic microenvironment for accelerating vascularized bone regeneration. Biomaterials. 2021;268:120561.
[7] GUPTA S, TEOTIA AK, QAYOOM I, et al. Periosteum-Mimicking Tissue-Engineered Composite for Treating Periosteum Damage in Critical-Sized Bone Defects. Biomacromolecules. 2021;22(8):3237-3250.
[8] FERNANDEZ DE GRADO G, KELLER L, IDOUX-GILLET Y, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng. 2018;9: 2041731418776819.
[9] DIMITRIOU R, JONES E, MCGONAGLE D, et al. Bone regeneration: current concepts and future directions. BMC Med. 2011;9(1):66.
[10] DWEK JR. The periosteum: what is it, where is it, and what mimics it in its absence? Skelet Radiol. 2010;39(4):319-323.
[11] LI N, SONG J, ZHU G, et al. Periosteum tissue engineering-a review. Biomater Sci. 2016;4(11):1554-1561.
[12] WANG Q, XU J, JIN H, et al. Artificial periosteum in bone defect repair-A review. Chin Chem Lett. 2017;28(9):1801-1807.
[13] HE X, LI W, LIU K, et al. Anisotropic and robust hydrogels combined osteogenic and angiogenic activity as artificial periosteum. Composit Part B Eng. 2022:233.
[14] QING Y, LI R, LI S, et al. Advanced Black Phosphorus Nanomaterials for Bone Regeneration. Int J Nanomedicine. 2020;15:2045-2058.
[15] ZHOU Y, ZHANG MX, GUO ZN, et al. Recent advances in black phosphorus-based photonics, electronics, sensors and energy devices. Mater Horiz. 2017;4(6):997-1019.
[16] LIU W, BI W, SUN Y, et al. Biomimetic organic-inorganic hybrid hydrogel electrospinning periosteum for accelerating bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110670.
[17] DOGANOV RA, O’FARRELL EC, KOENIG SP, et al. Transport properties of pristine few-layer black phosphorus by van der Waals passivation in an inert atmosphere. Nat Commun. 2015;6(1):6647.
[18] SUN Z, XIE H, TANG S, et al. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew Chem Int Ed Engl. 2015;54(39):11526-11530.
[19] ZHU C, XU F, ZHANG L, et al. Ultrafast Preparation of Black Phosphorus Quantum Dots for Efficient Humidity Sensing. Chemistry. 2016;22(22): 7357-7362.
[20] ZONG S, WANG L, YANG Z, et al. Black Phosphorus-Based Drug Nanocarrier for Targeted and Synergetic Chemophotothermal Therapy of Acute Lymphoblastic Leukemia. ACS Appl Mater Interfaces. 2019;11(6):5896-5902.
[21] UTT KL, RIVERO P, MEHBOUDI M, et al. Intrinsic Defects, Fluctuations of the Local Shape, and the Photo-Oxidation of Black Phosphorus. ACS Cent Sci. 2015;1(6):320-327.
[22] WU N, WANG XM, DAS CM, et al. Bioengineering applications of black phosphorus and their toxicity assessment. Environ Sci-Nano. 2021;8(12):3452-3477.
[23] ZHANG Y, MA C, XIE J, et al. Black Phosphorus/Polymers: Status and Challenges. Adv Mater. 2021;33(37):e2100113.
[24] DEVESCOVI V, LEONARDI E, CIAPETTI G, et al. Growth factors in bone repair. Chir Organi Mov. 2008;92(3):161-168.
[25] APTE RS, CHEN DS, FERRARA N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176(6):1248-1264.
[26] SARAN U, GEMINI PIPERNI S, CHATTERJEE S. Role of angiogenesis in bone repair. Arch Biochem Biophys. 2014;561:109-117.
[27] LEE SJ, KIM ME, NAH H, et al. Vascular endothelial growth factor immobilized on mussel-inspired three-dimensional bilayered scaffold for artificial vascular graft application: In vitro and in vivo evaluations. J Colloid Interface Sci. 2019;537:333-344.
[28] WANG XX, YU GF, ZHANG J, et al. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog Mater Sci. 2021:115.
[29] RAHMATI M, MILLS DK, URBANSKA AM, et al. Electrospinning for tissue engineering applications. Prog Mater Sci. 2021:117.
[30] ZHANG C, LI Y, WANG P, et al. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr Rev Food Sci Food Saf. 2020;19(2):479-502.
[31] YU DG, WANG M, LI X, et al. Multifluid electrospinning for the generation of complex nanostructures. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(3):e1601.
[32] HU X, LIU S, ZHOU G, et al. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12-21.
[33] YAZDANPANAH A, TAHMASBI M, AMOABEDINY G, et al. Fabrication and characterization of electrospun poly- L -lactide/gelatin graded tubular scaffolds: Toward a new design for performance enhancement in vascular tissue engineering. Prog Nat Sci Mater Int. 2015;25(5):405-413.
[34] LURAGHI A, PERI F, MORONI L. Electrospinning for drug delivery applications: A review. J Control Release. 2021;334:463-484.
[35] HE Y, LI X, MA J, et al. Programmable Codelivery of Doxorubicin and Apatinib Using an Implantable Hierarchical-Structured Fiber Device for Overcoming Cancer Multidrug Resistance. Small. 2019;15(8):e1804397.
[36] ASADI H, GHAEE A, NOURMOHAMMADI J, et al. Electrospun zein/graphene oxide nanosheet composite nanofibers with controlled drug release as antibacterial wound dressing. Int J Polym Mater. 2019;69(3): 173-185.
[37] ZHOU L, ZHU C, EDMONDS L, et al. Microsol-electrospinning for controlled loading and release of water-soluble drugs in microfibrous membranes. RSC Adv. 2014;4(81):43220-43226.
[38] WU L, GU Y, LIU L, et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227:119555.
[39] HU K, OLSEN BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126(2):509-526.
[40] STREET J, BAO M, DEGUZMAN L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656.
[41] SCHMITZ JP, HOLLINGER JO, MILAM SB. Reconstruction of bone using calcium phosphate bone cements: A critical review. J Oral Maxillofac Surg. 1999;57(9):1122-1126.
[42] JEONG J, KIM JH, SHIM JH, et al. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23:4.
[43] LEVINGSTONE TJ, HERBAJ S, DUNNE NJ. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials (Basel). 2019;9(11):1570.
[44] BOHNER M, GBURECK U, BARRALET JE. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials. 2005;26(33):6423-6429.
[45] WU S, HE F, XIE G, et al. Black Phosphorus: Degradation Favors Lubrication. Nano Lett. 2018;18(9):5618-5627.
[46] HUANG K, WU J, GU Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl Mater Interfaces. 2019;11(3):2908-2916.
[47] LEE YB, SONG SJ, SHIN YC, et al. Ternary nanofiber matrices composed of PCL/black phosphorus/collagen to enhance osteodifferentiation. J Ind Eng Chem. 2019;80:802-810.
[48] Zhao W, Xue Z, Wang J, et al. Large-Scale, Highly Efficient, and Green Liquid-Exfoliation of Black Phosphorus in Ionic Liquids. ACS Appl Mater Interfaces. 2015;7(50):27608-27612.
[49] ZILETTI A, CARVALHO A, TREVISANUTTO PE, et al. Phosphorene oxides: Bandgap engineering of phosphorene by oxidation. Phys Rev B. 2015;91(8):085407.
[50] BHARDWAJ N, KUNDU SC. Electrospinning: A fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325-347.
[51] DONG S, SUN J, LI Y, et al. Electrospun nanofibrous scaffolds of poly (l-lactic acid)-dicalcium silicate composite via ultrasonic-aging technique for bone regeneration. Mater Sci Eng C. 2014;35:426-433.
[52] EREN BONCU T, USKUDAR GUCLU A, CATMA MF, et al. In vitro and in vivo evaluation of linezolid loaded electrospun PLGA and PLGA/PCL fiber mats for prophylaxis and treatment of MRSA induced prosthetic infections. Int J Pharm. 2020;573:118758.
|