Chinese Journal of Tissue Engineering Research ›› 2015, Vol. 19 ›› Issue (6): 975-979.doi: 10.3969/j.issn.2095-4344.2015.06.027
Previous Articles Next Articles
Stem cells and stem cell therapy for diabetes mellitus: Chinese clinical trial registration information analysis
Liu Hong-juan
- Department of Endocrinology, Huludao Center Hospital, Huludao 125000, Liaoning Province, China
-
Received:2014-11-18Online:2015-02-05Published:2015-02-05 -
About author:Liu Hong-juan, Associate chief physician, Department of Endocrinology, Huludao Center Hospital, Huludao 125000, Liaoning Province, China
CLC Number:
Cite this article
Liu Hong-juan. Stem cells and stem cell therapy for diabetes mellitus: Chinese clinical trial registration information analysis[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(6): 975-979.
share this article
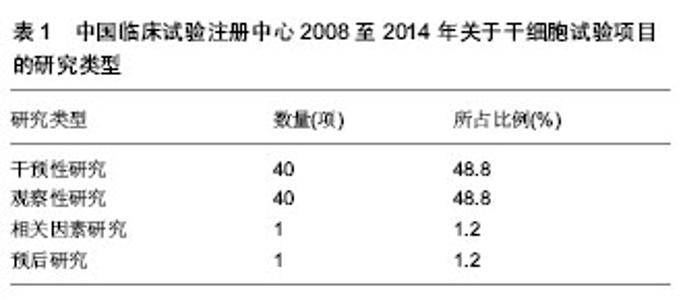
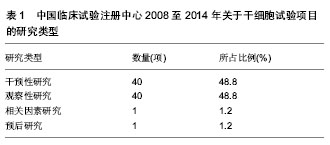
2.1 干细胞研究临床试验项目分析 在中国临床试验注册中心检索2008至2014年干细胞研究的相关数据,共有82项试验注册记录,从治疗疾病种类、研究类型、注册时间、注册单位、分布地区等方面对资料进行整理。 2.1.1 干细胞治疗疾病种类 2008至2014年中国临床试验注册中心关于干细胞研究的项目中,干细胞治疗疾病种类主要为血液病32项,占注册临床试验项目的39.0%,治疗神经系统疾病20项,占注册临床试验项目的24.4%、肝病和糖尿病分别为9项和5项,分别占注册临床试验项目的11.0%和6.1%,具体数据见图1。 2.1.2 临床试验的研究类型 2008至2014年中国临床试验注册中心关于干细胞研究的项目中,主要为干预性研究和观察性研究,临床因素研究和预后研究较少,具体数据见表1。"
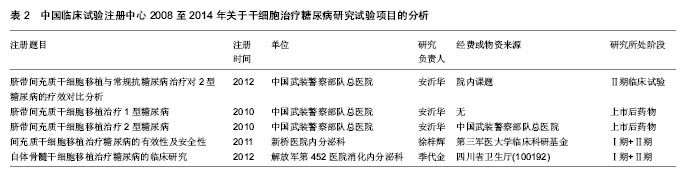
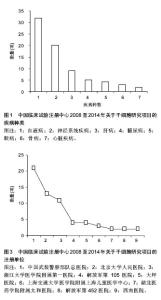
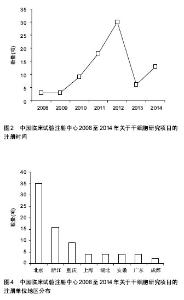
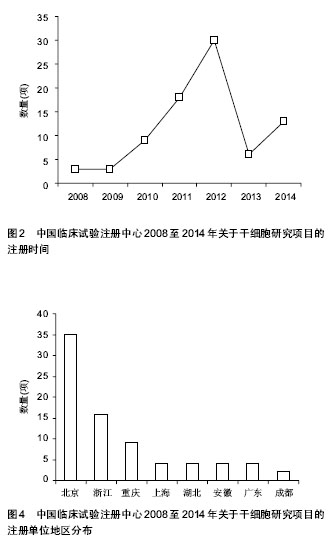
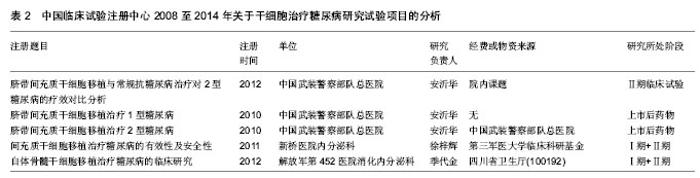
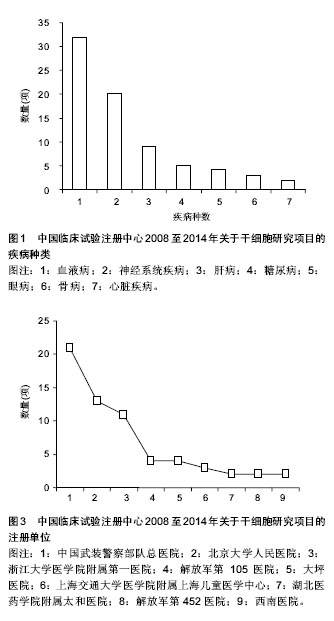
2.1.3 注册时间 2008至2014年中国临床试验注册中心关于干细胞研究的项目中,2012年注册项目数量最多为30项,占注册临床试验项目数量的36.6%,远高于2011年的18项,2014年注册临床试验项目数量13项,具体数据见图2。2012年注册项目的研究类型中,以观察性研究为主,数量为22项,干预性研究8项;治疗疾病种类主要有血液病14项、神经系统疾病9项、肝病3项和糖尿病2项;注册单位分别是北京大学人民医院8项,中国武装警察部队总医院7项,浙江大学医学院附属第一医院6项。 2.1.4 注册单位 2008至2014年中国临床试验注册数据关于干细胞研究的试验项目中,注册的研究单位主要为中国武装警察部队总医院(21/25.6%)、北京大学人民医院(13/15.9%)和浙江大学医学院附属第一医院(11/13.4%),具体数据见图3。中国武装警察部队总医院的临床试验注册项目中,治疗神经系统疾病的项目有15项,治疗糖尿病的项目有3项;以干预性研究为主,数量为13项,观察性研究8项;注册时间主要在2011年,有注册项目10项,2012年注册项目7项,2010年注册项目4项。 2.1.5 注册地区 2008至2014年中国临床试验注册数据关于干细胞研究的试验项目中,注册单位的地区主要分布在北京(35/42.7%)和浙江(16/19.5%),具体数据见图4。北京地区的临床试验注册单位主要是中国武装警察部队总医院19项和北京大学人民医院13项,浙江地区的临床试验注册单位是浙江大学医学院附属第一医院11项;北京地区治疗血液病的注册项目13项,治疗神经系统疾病的项目为12项,浙江地区以治疗血液病为主,注册项目有12项。 2.2 干细胞治疗糖尿病临床试验项目分析 在中国临床试验注册中心检索干细胞治疗糖尿病研究的试验项目有5项,详见表2。 有3项试验注册为脐带间充质干细胞移植治疗糖尿病的研究,注册单位为中国武装警察部队总医院,负责人是干细胞移植中心安沂华博士,注册时间在2010和2012年,Ⅱ期临床试验1项。研究内容为脐带间充质干细胞移植治疗1型和2型糖尿病。干细胞治疗2型糖尿病试验的纳入标准为年龄在18-70岁的患者,男女不限;血糖控制不佳已经3个月,无急性并发症倾向;诊断明确的2型糖尿病患者;空腹血糖≥7.0 mmol/L,糖化血红蛋白≥7%;心肝肾等脏器功能正常,能耐受介入等手术。治疗1型糖尿病患者的纳入标准除患者年龄外与2型糖尿病基本相同。排除恶性肿瘤、严重心肝肾功能不全、严重哮喘等呼吸功能不全的患者,排除有出血性疾病、凝血异常、造影剂过敏或正在应用抗凝药物的患者,先天性或获得性免疫缺陷疾病,有其他内分泌及代谢性疾病,有急慢性感染或妊娠糖尿病的患者。试验主要检测指标包括糖化血红蛋白、C肽、血糖、β细胞功能、胰岛素和抗糖尿病药物用量、免疫细胞检测、血清炎症因子(白细胞介素1β,白细胞介素6,白细胞介素10和肿瘤坏死因子α)等。 2011年,重庆新桥医院内分泌科徐梓辉教授注册的试验题目为“间充质干细胞移植治疗糖尿病的有效性及安全性”,属于干预性研究,预注册,试验处于Ⅰ期+Ⅱ期临床,目的是为了评估干细胞移植治疗糖尿病的有效性及安全性。试验纳入年龄在16-60岁的糖尿病患者,性别不限,合理应用胰岛素或口服血糖控制仍较差者,以及外源性胰岛素用量较大的患者,同意入选并签署知情同意书。排除患有肿瘤、HIV阳性、梅毒、活动性肝炎、心肝肾功不全、妊娠和有精神病以及内分泌相关等疾病的患者,试验分为药物治疗组和干细胞移植+药物治疗组,检测治疗前后胰岛素用量、血糖水平、体质量指数、糖化血红蛋白和C肽水平。 2012年,解放军第452医院消化内分泌科季代金注册项目名称为自体骨髓干细胞移植治疗糖尿病的临床研究,补注册,经费或物资来源于四川省卫生厅基金项目,试验处于Ⅰ期+Ⅱ期临床,研究纳入18-60岁明确诊断的2型糖尿病患者,性别不限,体质量指数在19- 24 kg/m2,糖化血红蛋白≥7.0%,患者签署知情同意书。"
| [1] De Angelis CD, Drazen JM, Frizelle FA, et al.Is this clinical trial fully registered?--A statement from the International Committee of Medical Journal Editors.N Engl J Med.2005; 352(23):2436-2438. [2] 晏小勇,陈永法.美国临床试验注册与结果公开制度研究[J].中国新药杂志,2011,20(7):577-581. [3] 高雪山,钟紫红.实行临床试验注册减少医学期刊发表偏倚[J].中国科技期刊研究,2009,20(5):854-857. [4] 李昕雪,韩梅,王禹毅,等.临床试验的国际注册及在美国临床试验注册平台注册的意义与方法[J].中医杂志,2013,54(19): 1640-1643. [5] Abaid LN, Grimes DA, Schulz KF. Reducing publication bias of prospective clinical trials through trial registration. Contraception. 2007;76(5):339-341. [6] Bonita RE, Adams S, Whellan DJ. Reporting of clinical trials: publication, authorship, and trial registration. Heart Fail Clin. 2011;7(4):561-567. [7] 严佳,裴建.临床试验注册在针灸临床的应用[J].中华针灸电子杂志,2014,3(1):8-12. [8] 李晓婷,张晓鹏,李艳玲,等.我国影像临床试验注册情况及其对科研管理的启示[J].中国医院管理,2013,33(9):62-64. [9] 曹莹莹,林能明,方罗.中国临床试验注册中心在册药物临床试验分析[J].中国药房,2012,23(41):3858-3860. [10] Lin HT, Kao CL, Lee KH, et al.Enhancement of insulin-producing cell differentiation from embryonic stem cells using pax4-nucleofection method.World J Gastroenterol. 2007;13(11):1672-1679. [11] Vaca P, Martín F, Vegara-Meseguer JM, et al.Induction of differentiation of embryonic stem cells into insulin-secreting cells by fetal soluble factors.Stem Cells.2006;24(2):258-265. [12] Nardo B, Masetti M, Urbani L, et al.Liver transplantation from donors aged 80 years and over: pushing the limit.Am J Transplant.2004;4(7):1139-1147. [13] Tang DQ, Wang Q, Burkhardt BR, et al.In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation.Am J Stem Cells. 2012;1(2): 114-127. [14] Gao F, Wu DQ, Hu YH, et al.Extracellular matrix gel is necessary for in vitro cultivation of insulin producing cells from human umbilical cord blood derived mesenchymal stem cells. Chin Med J (Engl). 2008;121(9):811-818. [15] Ngoc PK, Phuc PV, Nhung TH, et al.Improving the efficacy of type 1 diabetes therapy by transplantation of immunoisolated insulin-producing cells.Hum Cell.2011;24(2):86-95. [16] Gang EJ, Hong SH, Jeong JA, et al.In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells.Biochem Biophys Res Commun. 2004; 321(1):102-108. [17] Ito T, Itakura S, Todorov I, et al.Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function.Transplantation.2010;89(12):1438-1445. [18] Fleischmann E, Marschalek C, Schlemitz K, et al.Nitrous oxide may not increase the risk of cancer recurrence after colorectal surgery: a follow-up of a randomized controlled trial.BMC Anesthesiol. 2009;9:1. [19] Voils CI, Yancy WS Jr, Kovac S, et al. Study protocol: Couples Partnering for Lipid Enhancing Strategies (CouPLES) - a randomized, controlled trial. Trials. 2009;10:10. [20] Cima R, Joore M, Maes I, et al.Cost-effectiveness of multidisciplinary management of Tinnitus at a specialized Tinnitus centre.BMC Health Serv Res. 2009;9:29. [21] 季代金,黄茂涛.干细胞移植与糖尿病[J].临床军医杂志,2011, 39(1):168-171. [22] 季代金,黄茂涛,王维萍,等.自体骨髓干细胞治疗2型糖尿病2例[J].临床军医杂志,2011,39(1):199-200. [23] 姚金萍,段炼,童强,等.自体骨髓干细胞移植治疗2型糖尿病的安全性及有效性[J].第三军医大学学报,2012,34(1):74-77. [24] 隆敏,郑宏庭,童强,等.自体骨髓干细胞胰腺定向移植治疗糖尿病1例[J].第三军医大学学报,2010,32(6):524. [25] 郑培,安沂华,王晓东,等.糖尿病周围神经病变神经电生理特点分析[J].解放军医学院学报,2013,34(6):590-592,649. [26] 郑培,安沂华,王晓东,等.脐带间充质干细胞移植治疗糖尿病周围神经病变的疗效观察[J].武警医学,2013,24(5):398-401. [27] Liu X, Zheng P, Wang X, et al.A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus.Stem Cell Res Ther. 2014;5(2):57. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [14] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||