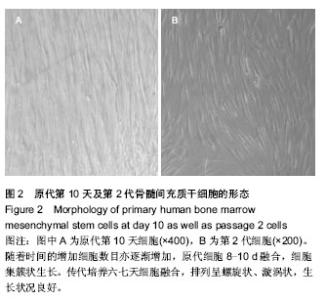
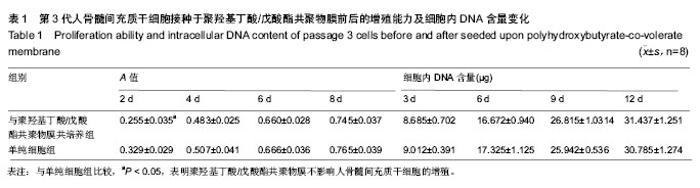
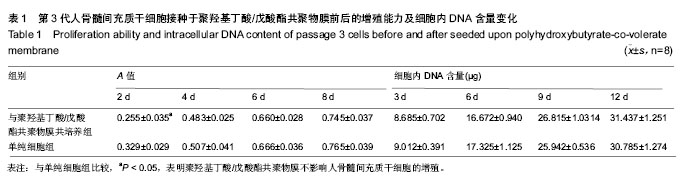
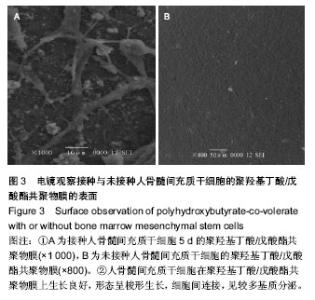
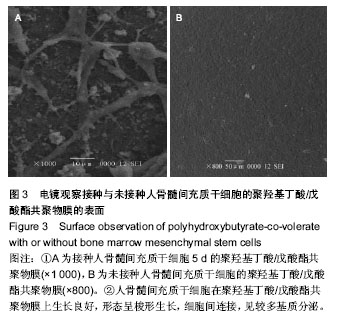
| [1] Chen GQ,Wu Q.The application of polyhydroxyalkanoates as tissue engineering materials.Biomaterials.2005;26: 6565-6578.
[2] Prabhakaran MP,Vatankhah E,Ramakrishna S,Electrospun Aligned PHBV/Collagen Nanofibers as Substrates for Nerve Tissue Engineering. Biotechnol Bioeng,2013;110(10):2775- 2784.
[3] Kose GT,Korkusuz F,Ozkul A,et al.Tissue engineered cartilage on collagen and PHBV matrices.Biomaterials. 2005; 26(25):5187-5197.
[4] 周栋,汪钢,赵东锷,等.应用聚羟基丁酸/聚羟基戊酸共聚物构建组织工程心脏瓣膜的实验研究[J].中国胸心血管外科临床杂志,2004,11(2):116-119.
[5] 唐倩,李源,梁焕友,等.聚羟基丁酸/羟基戊酸共聚酯构建引导骨组织再生膜的实验研究[J].中国现代医学杂志,2007,17(9): 1046-1050.
[6] Vilos C,Morales FA,Solar PA,et al.Paclitaxel-PHBV nanoparticles and their toxicity to endometrial and primary ovarian cancer cells.Biomaterials.2013;34:4098-4108.
[7] Ahmed TAE,Giulivi A,Griffith M,et al.Fibrin Glues in Combination with Mesenchymal Stem Cells to Develop a Tissue-Engineered Cartilage Substitute.Tissue Eng Part A.2011;17(3-4):323-335.
[8] Gao JZ,Dennis JE,Solchaga LA,et al.Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng,2001; 7(4):363-371.
[9] Szpalski C,Barbaro M,Sagebin F,et al.Bone Tissue Engineering: Current Strategies and Techniques-Part II: Cell Types.Tissue Eng Part B-Rev.2012;18(4):258-269.
[10] Xie J,Han ZY,Naito M,et al.Articular cartilage tissue engineering based on a mechano-active scaffold made of poly(L-lactide-co-epsilon-caprolactone): In vivo performance in adult rabbits.J Biomed Mater Res Part B Appl Biomater. 2010; 94B(1):80-88.
[11] 赵大成,汪玉良,党跃修,等.骨髓间充质干细胞在组织工程研究中的应用进展[J].中国组织工程研究与临床康复,2011,15(49): 9271-9274.
[12] 张凯,王毅,邢国胜.间充质干细胞的生物学特性及多向分化潜能[J].中国组织工程研究与临床康复,2008,12(3):539-542.
[13] 刘伟,刘萌,祝劲松,等.人骨髓间充质干细胞的体外培养、鉴定及成骨分化[J].中国组织工程研究,2012,16(14):2515-2519.
[14] Frith JE,Mills RJ,Hudson JE,et al.Tailored Integrin-Extracellular Matrix Interactions to Direct Human Mesenchymal Stem Cell Differentiation.Stem Cells Dev. 2012; 21(13):2442-2456.
[15] Vickers SM,Gotterbarm T,Spector M.Cross-Linking Affects Cellular Condensation and Chondrogenesis in Type II Collagen-GAG Scaffolds Seeded with Bone Marrow-Derived Mesenchymal Stem Cells.J Orthop Res. 2010;28(9):1184-1192.
[16] Xue JX,Gong YY,Zhou GD,et al.Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets.Biomaterials. 2012; 33(24):5832-5840.
[17] Bhardwaj N,Kundu SC.Chondrogenic differentiation of rat MSCs on porous scaffolds of silk fibroin/chitosan blends. Biomaterials.2012;33(10):2848-2857.
[18] Langer R,Vacanti JP.Tissue engineering.Science. 1993; 260(5110):920-926.
[19] Stock UA,Vacanti JP.Tissue engineering: current state and prospects.Annu Rev Med. 2001;52:443-451.
[20] Liu C,Xia Z,Czernuszka JT.Design and development of three-dimensional scaffolds for tissue engineering.Chem Eng Res De.2007;85(7):1051-1064.
[21] Lannutti J,Reneker D,Ma T,et al.Electrospinning for tissue engineering scaffolds. Mat Sci Eng C.2007;27:504-509.
[22] Murugan R,Ramakrishna S.Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng.2007;13(8):1845-1866.
[23] Martins AM,Alves CM,Kasper FK,et al.Responsive and in situ-forming chitosan scaffolds for bone tissue engineering applications: an overview of the last decade. J Mater Chem. 2010;20 (9):1638-1645.
[24] 章柏平,汤善华,张立,等.可塑性纳米羟基磷灰石/聚羟基丁酸酯-羟基戊酸酯共聚物-聚乙二醇释药系统治疗兔骨髓炎[J].中国组织工程研究与临床康复,2011,15(21):3871-3875.
[25] Kose GT,Korkusuz F,Korkusuz P,et al.Bone generation on PHBV matrices: An in vitro study.Biomaterials. 2003;24: 4999-5007.
[26] Liu J,Zhao B,Zhang YQ,et al.PHBV and predifferentiated human adipose-derived stem cells for cartilage tissue engineering.J Biomed Mater Res A.2010;94(2):603-610.
[27] Morent R,Geyter ND,Desmet T,et al.Plasma surface modification of biodegradable polymers: a review.Plasma Process Polym.2011;8(3):171-190.
[28] Lao HK,Renard E,Linossier I,et al.Modification of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) film by chemical graft copolymerization. Biomacromolecules. 2007; 8(2):416-423.
[29] Wang YY,Lu LX,Shi JC,et al.Introducing RGD peptides on PHBV films through PEG-containing cross-linkers to improve the biocompatibility. Biomacromolecules.2011; 12(3): 551-559.
[30] Bakare RA,Bhan C,Raghavan D.Synthesis and characterization of collagen grafted Poly(hydroxybutyrate-valerate) (PHBV) scaffold for loading of bovine serum albumin capped silver (Ag/BSA) nanoparticles in the potential use of tissue engineering application.Biomacromolecules.2014;15(1):423-435.
[31] Wang Y,Ke Y,Wang L,et al.Preparation of amide-amine bifunctionalized poly(3-hydroxybutyrate-co-3- hydroxyvalerate) films to improve chondrocyte adhesion.J Biomater Sci Polym Ed.2009;20(5-6):673-687.
[32] Kuppan P,Vasanthan KS,Sundaramurthi D,et al. Development of poly(3-hydroxybutyrate-co-3- hydroxyvalerate) fibers for skin tissue engineering: effects of topography, mechanical, and chemical stimuli. Biomacromolecules.2011; 12(9):3156-3165.
[33] Kuppan P, Sethuraman S, Krishnan UM.Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-based nanofibrous scaffolds to support functional esophageal epithelial cells towards engineering the esophagus.J Biomater Sci Polym Ed.2014 Feb 6. [Epub ahead of print]
[34] Zhu XH,Wang CH,Tong YW.In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold.J Biomed Mater Res A.2009; 89(2): 411-423.
[35] Han I,Shim KJ,Kim JY,et al.Effect of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanofiber matrices cocultured with hair follicular epithelial and dermal cells for biological wound dressing.Artif Organs.2007; 31(11): 801-808.
[36] Tang S,Jin A,Wang Y.Preparation of plastic nano-hydroxyapatite/poly (3-hydroxybutyrate- hydroxyvalerate)-polymethyl lene glycol gentamicin drug delivery system.Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.2006;20(7):758-761.
[37] 张乃丽,李宝兴,赵亚平,等.生物衍生骨材料与骨髓间充质干细胞体外的生物相容性[J]. 中国组织工程研究与临床康复, 2008,12(49):9766-9770. |