Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (3): 501-508.doi: 10.3969/j.issn.2095-4344.2013.03.020
Previous Articles Next Articles
Synthetic polymer scaffolds for spinal cord injury
Kan Rui, Sheng Wei-bin
- Department of Spinal Surgery, the First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China
-
Received:2012-07-19Revised:2012-08-12Online:2013-01-15Published:2013-02-25 -
Contact:Sheng Wei-bin, Doctor, Professor, Chief physician, Doctoral supervisor, Department of Spinal Surgery, the First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China wbsheng@vip.sina.com -
About author:Kan Rui★, Studying for master’s degree, Physician, Department of Spinal Surgery, the First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China kan-rui@126.com
CLC Number:
Cite this article
Kan Rui, Sheng Wei-bin. Synthetic polymer scaffolds for spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(3): 501-508.
share this article

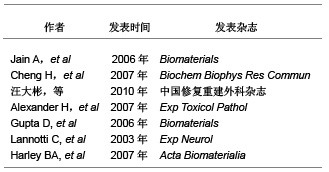
2.1 支架材料在脊髓损伤中的研究现状 目前的支架材料主要有以下几类:天然支架材料、人工合成高分子支架材料和其他支架材料。天然支架材料主要有壳聚糖、纤维蛋白、葡聚糖、胶原、凝胶等[5]。Cheng等[6]设计的多孔壳聚糖支架用于治疗大鼠脊髓损伤,观察到该支架能使大鼠脊髓功能部分恢复。也有学者证实壳聚糖-藻盐酸支架,具有潜在的应用前景[7]。Alexander等[8]证明纤维蛋白支架材料可促进脊髓损伤恢复,从鲑鱼体内提取的纤维蛋白可促进神经元的再生。除此之外,透明质酸、胶原和基质明胶等也可促进脊髓损伤的修复[9-11]。虽然这些天然的支架材料均可促进脊髓损伤的恢复,但是他们的生物力学特性和正常脊髓组织有着非常大的差别,因而这些天然高分子材料不能作为理想的脊髓支架材料。 文章选取的支架材料在脊髓损伤中应用的相关研究:"
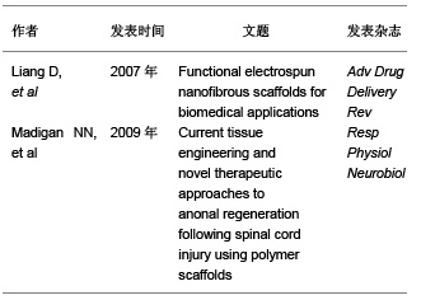
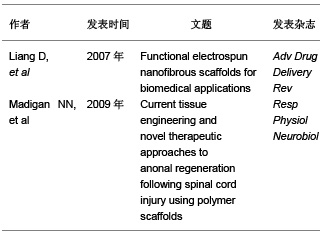
2.2 支架材料的要求 脊髓损伤后会导致损伤区域的细胞和组织丢失,损伤后的神经保护策略并不能阻止其病变发展。目前认为组织工程支架可以模拟细胞外基质的生理状态,促进细胞的黏附、迁移、扩增和分化[12]。因此理想的支架材料应达到以下要求[13]:①具有良好的生物生物相容性:有免疫保护作用或无免疫排斥作用,不会引起炎性反应等。②良好的降解性:植入后能适时讲解,并与组织细胞相适应。③良好的细胞接触面:为种子细胞提供生长的框架和附着点。④能引导细胞的迁移、增值、分化、表型和凋亡。⑤能防止胶质瘢痕的形成。⑥具有高空隙率:将有利于细胞的植入和植入。⑦高通透性:将有利于营养物质和神经营养因子通透。⑧可塑性和强度:在体外便于塑形,植入后能保持一定时间。 文章选取的有关脊髓损伤对支架要求的研究:"
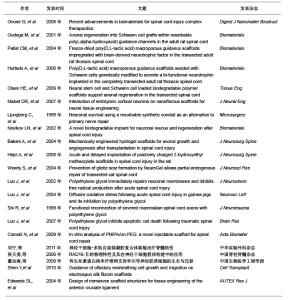
2.3 人工合成高分子支架材料的研究现状 脊髓损伤后神经轴突有一定的再生潜能,这对脊髓损伤的治疗奠定了理论基础,但损伤区域细胞、组织的破坏等一系列因素使胶质瘢痕形成和发展,其微环境不利于轴突再生修复,使脊髓功能的恢复变得异常困难。目前支架材料在治疗脊髓损伤中的应用主要是通过种子细胞与支架材料构成细胞-支架复合体来治疗脊髓损伤,在人体组织中细胞指导细胞外基质的合成,细胞外基质又可促进细胞的迁移、增殖、分化、表型和凋亡,二者相互作用、相互影响,细胞外基质又防止胶质瘢痕的形成,因而支架的构建是治疗脊髓的关键。因为天然高分子支架材料的生物力学特性和正常脊髓组织有着非常大的差别,因此,目前的科研工作者集中研究人工合成高分子支架材料。 2.3.1 人工合成聚乳酸 聚乳酸是乳酸的聚合物,是可再生资源,植入体内后其代谢具有无毒行,因而在临床大量应用[14]。聚乳酸具有很多优点:生物相容性和组织降解性很好;具有良好地机械性能;可作为细胞基质使用。有学者证实聚乳酸在脊髓内也具有较好的生物相容性。Oudega等[15]通过实验证明聚乳酸和许旺细胞联合移植可显著改善大鼠后肢功能,促进轴突的再生。Patist等[16]将大孔聚乳酸支架联合细胞移植到受损脊髓中,观察到其与脊髓神经组织具有更好的生物相容性,并观察到大孔支架有提高种子细胞的存活率。Hurtado等[17]将聚乳酸支架联合细胞移植,同样观察到大鼠全切模型后肢运动功能的改善。无论是单独聚乳酸移植,还是联合细胞移植,聚乳酸作为支架材料仍没有根本解决脊髓损伤后的修复问题。 2.3.2 聚羟基酸 聚羟基酸是人工合成的另一种高分子可降解的聚合物,主要通过控制聚合物的分子质量及其组成成分调控它们的生物力学特性和降解速率。目前以聚羟基乙酸和聚乳酸-羟基乙酸共聚物作为人工合成的支架材料。其优点是:人工合成;变异性低,可体内降解;机械和物理性能可控制,重复性好;材料制备纯度高。Olson等[18]将神经干细胞和雪旺细胞作为种子细胞种植于聚乳酸-羟基乙酸共聚物支架上,然后移植于脊髓横断的小鼠,发现种子细胞存活并有促进轴突再生的作用。也有学者通过改变支架张力提高支架材料对神经再生的作用。Nisbe等[19]通过氢氧化钾部分水解聚羟基乙酸和聚乳酸-羟基乙酸共聚物支架,以改变其表面张力,发现当两种支架的表面张力为0.40-0.47 mN/cm时,神经轴突再生延伸最显著,这提示接触诱导在引导轴突再生方面起重要作用。 文章选择的人工合成高分子支架材料在脊髓损伤中应用的相关研究:"
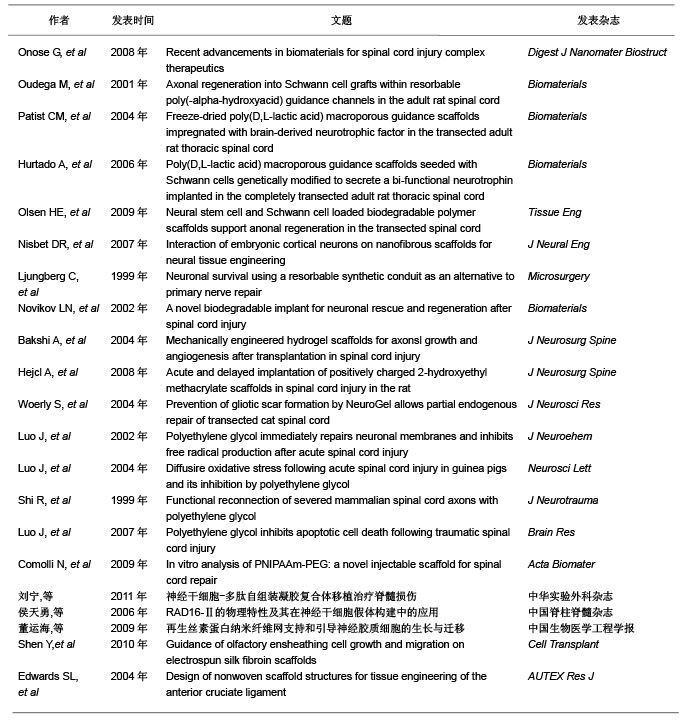

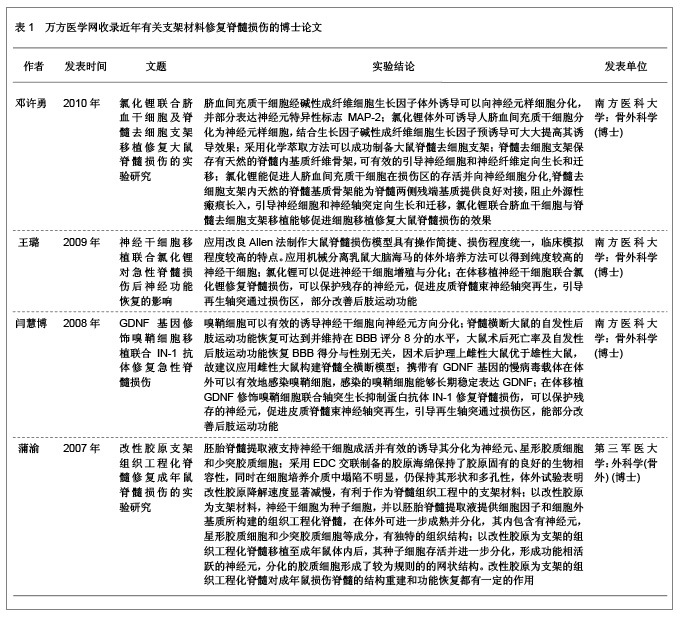
2.3.3 聚β-羟丁酸 聚β-羟丁酸是新近人工合成的高分子支架材料,不仅具有生物相容性和在体内可降解性,并且在植入体内后通过体温的影响缓慢降解,其代谢产物无毒性,通过尿液代谢。聚β-羟丁酸是最早用于周围神经损伤的支架材料[20],取得了较好的疗效。 Novikova等[21]将聚β-羟丁酸纤维制成可降解微导管,并使其在2个垂直平面平行排列,形成片状结构,以此构建中枢神经支架;随后将藻酸盐水凝胶、纤连蛋白联合有许旺细胞的聚β-羟丁酸支架移植到损伤脊髓处,与对照组相比较胶质瘢痕形成减少,瘢痕空洞也减少。 2.3.4 合成水凝胶 合成水凝胶主要有聚甲基丙烯酸β-羟丙酯水凝胶、聚羟乙基丙烯酸甲酯和聚羟乙基丙烯酸甲酯-甲基丙烯酸甲酯共聚物,其特点为:亲水性的高分子材料聚合交联形成,含水量高、表面积大;在体内无毒性,属于惰性高分子聚合物;体内不降解性,可以为细胞提供粘附及轴突生长的三维支架结构。合成水凝胶具有的网络结构和低表面张力,能够携带小分子物质(如各种细胞生长因子),并能制成与脊髓同样力学性能的支架,植入损伤脊髓后生物相容性好,并能促进脊髓轴突再生长入支架内部[22]。 Hejcl等[23]通过实验发现,脊髓损伤后1周移植聚羟乙基丙烯酸甲酯支架,能够减少脊髓囊腔的形成,促进再生轴突生长。聚甲基丙烯酸β-羟丙酯和聚羟乙基丙烯酸甲酯都能够抑制脊髓损伤后胶质瘢痕的形成,提供脊髓轴突再生及髓鞘化的适宜微环境[24]。这种材料的优点是可以改变其物理和化学特性以适应脊髓修复的需要。 2.3.5 聚乙二醇 聚乙二醇是水溶性的聚合物,其特点为抑制自由基的产生,有阻止脂质过氧化反应的作用。将其移植于损伤脊髓区域,发现其有逆转因损伤引起的细胞膜通透性增加的作用[25-26]。Shi等[27]将聚乙二醇植入猪完全横断的脊髓损伤区域,发现其能够重建脊髓解剖结构的连续性,促进脊髓功能恢复。Luo等[28]报道聚乙二醇能够显著降低细胞凋亡蛋白酶3的活性,减少细胞程序性死亡,聚乙二醇与线粒体相互作用能提高线粒体的功能,减少细胞凋亡因子细胞色素C的释放,认为聚乙二醇是通过与线粒体相互作用抑制细胞程序性死亡,聚乙二醇主要通过修复损伤的细胞膜,减少细胞死亡和保护线粒体功能抑制细胞凋亡,在脊髓损伤再生修复方面起到十分重要的作用。 2.3.6 其他新型合成支架材料 除上述人工合成的高分子支架材料外,也学者通过体外实验制备了可注射支架材料-异丙基丙烯酰胺-乙二醇共聚物,这种支架材料能够持续4周左右释放神经营养因子,其力学性能与正常脊髓组织相适应,并与骨髓间充质干细胞有良好的相容性,是可以用于脊髓损伤修复的理想支架材料[29]。 刘宁等[30]构建的异亮氨酸-赖氨酸-缬氨酸-丙氨酸-缬氨酸支架材料与神经干细胞联合移植治疗脊髓损伤,观察到此种支架材料不仅促进了神经干细胞向神经元分化,而且促进脊髓损伤的恢复。侯天勇等[31]构建的精氨酸-丙氨酸-天门冬氨酸按照can-RARADADARARADA-DA-CNH2顺序组成的16个氨基酸短肽联合骨髓间充质干细胞治疗脊髓损伤,发现此支架材料能够提高神经干细胞的迁移、增殖和分化,认为此种支架材料在脊髓损伤的修复中可能具有潜在的应用前景。也有学者研究了神经胶质细胞在丝素蛋白纳米纤维网上的生长与迁移,结果表明纳米级纤维状丝素材料可很好地支持星形胶质细胞的黏附、生长、增殖及迁移[32]。 Shen等[33]研究了丝素蛋白纳米纤维对嗅鞘细胞生长及迁移引导的作用,结果表明丝素蛋白纳米纤维与组织工程支架材料在脊髓损伤修复中的应用具有良好的生物相容性,对嗅鞘细胞生长迁移起重要的支持引导作用,同时对细胞神经营养因子基因和蛋白的表达没有任何不良影响。因此,可以展望,丝素蛋白纳米纤维有望成为修复脊髓损伤的理想组织工程支架材料,而联合细胞移植更是有待于深入研究[34]。 2.4 万方医学网近年有关人工高分子材料修复脊髓损伤的相关学位论文 邓许勇探讨了脐血间充质干细胞体外分离、纯化、扩增的方法与条件。以及向神经细胞定向诱导分化的可行性,为脐血干细胞移植治疗脊髓损伤提供理论依据;探讨了氯化锂体外诱导人脐血间充质干细胞向神经细胞分化的可行性;制备脊髓去细胞支架,观测脊髓内基质骨架的结构特点;探索了氯化锂联合脐血干细胞及脊髓去细胞支架移植对大鼠脊髓全横断损伤的效果及其机制。 王璐应用改良Allen法制造大鼠急性脊髓损伤模型,体外培养神经干细胞并观察氯化锂对其增殖分化的影响,并将神经干细胞与氯化锂二者结合起来对急性脊髓损伤进行联合干预,观察神经干细胞移植与氯化锂联合干预对于脊髓损伤的修复效果,并探讨其作用机制,为临床进一步应用提供理论依据。 闫慧博拟应用GDNF基因修饰嗅鞘细胞移植和微泵局部定点持续给予轴突生长抑制蛋白抗体IN-1来修复脊髓损伤,观察该方法对脊髓损伤后神经再生和脊髓修复的影响,并探讨其促进脊髓修复的可能作用机制。 蒲渝拟体外构建天然支架材料胶原并对其进行交联改性,并通过支架材料的性能测试和生物相容性研究与人工合成材料聚乙醇酸,聚乳酸-乙醇酸进行比较。然后选择脊髓源性神经干细胞作为种子细胞接种在筛选出来的支架材料上,再以胚胎脊髓悬浮液提供神经干细胞生长分化所需的细胞因子和细胞外基质,以期构建组织工程化脊髓用于移植修复成年大鼠脊髓损伤。 结果见表1。"
| [1] Jurga M,Dainiak MB, Sarnowska A, et al. The perfortmmce of laminincontaining cryogel scaffolds in neural tissue regeneration. Biomaterials. 2011; 32(13): 3423-3434.[2] Wllerth SM,Saldy-Ebert SE.Appmaches to neural tissue engineering using scaffolds for drug delivery. Adv Drug Delive.2007;59(4-5):325-338.[3] Meek MF,Coert JH.Clinical use of nerve conduits in peripheral-nerve repair:review of the literature. J Reconstr Microsurg.2002;18(2)97-109.[4] Straley KS,Foo CW,Heilshom SC.Biomaterial design strategies for the treatrment of spinal cord injuries. J Neurotrauma.2010;27(1):1-19.[5] Jain A,Kim YT,McKeon RJ,et al.In situ gelling hydrogels for conformal repair of spinal cord defects and local delivery of BDNF after spinal cord injury. Biomaterials.2006; 27(3):497-504.[6] Cheng H,Huang YC,Chang PT,et al.Laminin-incorporated nerve conduits made by plasma treatment for repairing spinal cord injury. Biochem Biophys Res Commun.2007;357(4): 938-944.[7] 汪大彬,文益民,蓝旭,等. 壳聚糖-藻酸盐支架复合BMSCs修复急性脊髓损伤的实验研究[J].中国修复重建外科杂志,2010, 24(2):190-196.[8] Alexander H,Puchner P,Froetscher W,et al.The long-term neurocompatibility of human fibrin sealant and equine collagen as biomatrices in experimental spinal cord injury.Exp Toxicol Pathol.2007;58(4):237-245.[9] Gupta D,Tator CH,Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord.Biomaterials.2006;27(11): 2370-2379.[10] Lannotti C,Li H,Yan P,et al.Glial cell line derived neurotrophic factor enriched bridging rransplants promote propriospinal anonal regeneration and enhance myelination after spinal cord injury. Exp Neurol.2003;183(2):397-393.[11] Harley BA,Leung JH,Silva EC,et al.Mechanical characterization of collagen glycosaminoglycan scaffolds. Acta Biomaterialia.2007;4(3):463-474.[12] Liang D,Hsiao BS,Chu B. Functional electrospun nanofibrous scaffolds for biomedical applications.Adv Drug Delivery Rev.2007;59(14):1392-1412.[13] Madigan NN,McMahon S,Obrien T,et al. Current tissue engineering and novel therapeutic approaches to anonal regeneration following spinal cord injury using polymer scaffolds. Resp Physiol Neurobiol.2009;169(2):183-199.[14] Onose G,Ciureaa AV,Rizeaa RE,et al. Recent advancements in biomaterials for spinal cord injury complex therapeutics. Digest J Nanomater Biostruct. 2008;2(4): 307-314.[15] Oudega M,Gautier SE,Chapon P,et al.Axonal regeneration into Schwann cell grafts within resorbable poly(-alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials.2001;22(10):1125-1136.[16] Patist CM,Mulder MB,Gautier SE,et al.Freeze-dried poly(D,L-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord.Biomaterials. 2004;25(9):1569-1582.[17] Hurtado A,Moon LD,Maquet V,et al.Poly(D,L-lactic acid) macroporous guidance scaffolds seeded with Schwann cells genetically modified to secrete a bi-functional neurotrophin implanted in the completely transected adult rat thoracic spinal cord.Biomaterials.2006;27(3):430-442.[18] Olsen HE,Rooney GE,Gross L,et al.Neural stem cell and Schwann cell loaded biodegradable polymer scaffolds support anonal regeneration in the transected spinal cord. Tissue Eng.2009;15(7):1797-1805. [19] Nisbet DR,Pattanawong S,Ritchie NE,et al.Interaction of embryonic cortical neurons on nanofibrous scaffolds for neural tissue engineering. J Neural Eng.2007;4(2):35-41.[20] Ljungberg C,Johansson-Ruden G,Bostrom KJ,et al.Neuronal survival using a resorbable synthetic conduit as an alternative to primary nerve repair. Microsurgery.1999;19(6):259-264.[21] Novikov LN,Novicova LN,Mosahebi A,et al.A novel biodegradable implant for neuronal rescue and regeneration after spinal cord injury. Biomaterials.2002; 23(16):3369-3376.[22] Bakshi A,Fisher O,Dagci T,et al.Mechanically engineered hydrogel scaffolds for axonsl growth and angiogenesis after transplantation in spinal cord injury.J Neurosurg Spine.2004;1(3):32-329.[23] Hejcl A,Urdzikova L,Sedy J,et al.Acute and delayed implantation of positively charged 2-hydroxyethyl methacrylate scaffolds in spinal cord injury in the rat.J Neurosurg Spine.2008;8(1):67-73.[24] Woerly S,Doan VD,Sosa N,et al.Prevention of gliotic scar formation by NeuroGel allows partial endogenous repair of transected cat spinal cord. J Neurosci Res.2004;75(2): 262-272.[25] Luo J,Borgens R,Shi R.Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neuroehem.2002;83(2):471-480.[26] Luo J,Shi R. Diffusire oxidative stress following acute spinal cord injury in guinea pigs and its inhibition by polyethylene glycol.Neurosci Lett.2004;359(3):167-170.[27] Shi R,Borgens RB,Blight AR.Functional reconnection of severed mammalian spinal cord axons with polyethylene glycol. J Neurotrauma.1999;16(8):727-738.[28] Luo J,Shi R.Polyethylene glycol inhibits apoptotic cell death following traumatic spinal cord injury. Brain Res.2007;1155:10-16.[29] Comolli N,Neuhuber B,Fischer I,et al.In vitro analysis of PNIPAAm-PEG: a novel injectable scaffold for spinal cord repair.Acta Biomater.2009;5(4):1046-1055.[30] 刘宁,郭海龙,盛伟斌,等. 神经干细胞-多肽自组装凝胶复合体移植治疗脊髓损伤[J].中华实验外科杂志,2011,28(11):1961-1963.[31] 侯天勇,伍亚民,龙在云, 等.RAD16-Ⅱ的物理特性及其在神经干细胞假体构建中的应用[J].中国脊柱脊髓杂志,2006,16(10): 781-784.[32] 董运海,张峰,左宝齐,等.再生丝素蛋白纳米纤维网支持和引导神经胶质细胞的生长与迁移[J].中国生物医学工程学报,2009, 28(1) : 96-102.[33] Shen Y,Qian Y,Zhang HX.Guidance of olfactory ensheathing cell growth and migration on electrospun silk fibroin scaffolds.Cell Transplant.2010;19(2):147-157.[34] Edwards SL,Mitchell W,Matthews JB,et al.Design of nonwoven scaffold structures for tissue engineering of the anterior cruciate ligament. AUTEX Res J.2004;4(2):86-94.[35] Guo SZ,Ren XJ,Wu B,et al.Preparation of the acellular scaffold of the spinal cord and the study of biocompatibility. Spinal Cord.2010;48(7):576-581. |
| [1] | Liu Yang, Liu Donghui , Xu Lei, Zhan Xu, Sun Haobo, Kang Kai. Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2072-2080. |
| [2] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [3] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [4] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [5] | Wang Mingqi, Feng Shiya, Han Yinhe, Yu Pengxin, Guo Lina, Jia Zixuan, Wang Xiuli. Construction and evaluation of a neuralized intestinal mucosal tissue engineering model in vitro [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 892-900. |
| [6] | Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948. |
| [7] | Yang Hu, Zheng Yu, Jia Chengming, Wang Tong, Zhang Guangfei, Ji Yaoyao. Immune microenvironment regulates bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 701-710. |
| [8] | Wang Zhuo, Sun Panpan, Cheng Huanzhi, Cao Tingting. Application of chitosan in repair and regeneration of oral hard and soft tissues [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 459-468. |
| [9] | Wang Yu, Fan Minjie, Zheng Pengfei. Application of multistimuli-responsive hydrogels in bone damage repair: special responsiveness and diverse functions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 469-479. |
| [10] | Yan Qiquan, Yang Libin, Li Mengjun, Ni Yazhuo, Chen Keying, Xu Bo, Li Yaoyang, Ma Shiqing, Li Rui, Li Jianwen. Preparation and antibacterial properties of porcine small intestinal submucosal composite nanohydroxyapatite bioscaffold loaded with antimicrobial peptide KR-12-a5 [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 384-394. |
| [11] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [12] | Gu Jianmei, Yuan Kunshan, Zhou Qiang, Zhang Haijun, , . Application of laser microporous decellularized scaffolds in tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 499-507. |
| [13] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| [14] | Xu Wenhe, Li Xiaobing, Liu Fang. Functionalized biomimetic mineralized collagen modified orthopedic implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 516-527. |
| [15] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

