Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (30): 4896-4903.doi: 10.12307/2023.559
Previous Articles Next Articles
Application of poly(lactic-co-glycolic acid) copolymer microspheres in bone tissue engineering
Zhang Xiaoyu, Chen Qi, Yang Xing, Hao Yuefeng
- Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, Jiangsu Province, China
-
Received:2022-09-08Accepted:2022-10-28Online:2023-10-28Published:2023-04-03 -
Contact:Hao Yuefeng, MD, Professor, Chief physician, Doctoral supervisor, Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, Jiangsu Province, China -
About author:Zhang Xiaoyu, Master candidate, Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, Jiangsu Province, China -
Supported by:Natural Science Foundation of Jiangsu Province, No. BK20211083 (to HYF); Medical Key Discipline Project of Suzhou, No. SZXK202111 (to HYF)
CLC Number:
Cite this article
Zhang Xiaoyu, Chen Qi, Yang Xing, Hao Yuefeng. Application of poly(lactic-co-glycolic acid) copolymer microspheres in bone tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4896-4903.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

2.1 PLGA性质对微球影响 PLGA是由乳酸(lactic acid,LA)和羟基乙酸(glycolic acid,GA)按不同比例聚合而成的一类生物高分子材料,乳酸和羟基乙酸单体通过酯键连接,形成线性、无定形的脂肪族聚酯,其合成方法可分为两类:①乳酸和羟基乙酸直接聚合,这种方法得到的PLGA通常分子质量较低、相对分子质量分布较广;②丙交酯和乙交酯开环聚合,该方法得到的产物相对分子质量更高、更均匀[7]。通过调节单体比(乳酸/羟基乙酸)、分子质量、浓度和端基团等可以改变PLGA的性质,影响PLGA微球的包封率和药物释放动力学[9]。 PLGA保留了乳酸和羟基乙酸的一些性质,如乳酸的刚性、疏水性和缓慢降解性以及羟基乙酸的延伸性、相对较小的疏水性和较快的降解性[10]。一般来说,提高羟基乙酸的单体含量可以增加聚合物的亲水性,但是聚合物结构的无定形程度也会相应增加[11-12]。既往研究表明,乳酸和羟基乙酸比例分别为85∶15和75∶25的PLGA降解周期分别为8周和16周。当两种单体的比例为50∶50时,PLGA微球的降解速度最快,这是因为乳酸/羟基乙酸比为50∶50的PLGA结晶度最低,亲水性最高,有助于水渗透到聚合物基质中,加快降解速度[13]。此外,较高的乳酸/羟基乙酸比会增加微球的表面粗糙度[14],但降低了微球的表面孔隙率[15]。 分子质量较高的PLGA聚合物有较长的聚合物链,因此需要更长的降解时间,从而导致较慢的释放速率[16]。在骨组织工程中,出于对于载药和细胞生长的考虑,经常选用相对分子质量75 000-100 000的PLGA来制备微球。各种研究也证实了PLGA颗粒的粒径对自身降解和药物释放的影响。增加PLGA的浓度会增加微球的粒径[17],而大颗粒比小颗粒的突释少[18],因为它们有更长的扩散路径长度。而且大的PLGA微球由于具有更高的载药量和药物诱导的多孔结构,增加了药物的释放倾向,从而提供了更高的药物扩散速度[19]。 除了不同的共聚比和相对分子质量外,PLGA的类型还可以根据可用的自由端基分为端羟基、端羧基和端酯基。到目前为止,已有一些文献报道端基团可以引起聚合物性质的不同,从而进一步影响微球的性质,特别是对包封率和释放动力学的影响。不同端基团的PLGA,亲水性和降解率不同。一般来说,端羧基的PLGA更亲水[20],端羟基和端酯基的PLGA具有更好的耐水解性,降解周期更长[21]。端羟基或端酯基的PLGA制备的微球包封率低于端羧基微球,体外释药速度快于端羧基微球[9],端羧基微球包裹的药物具有相对较低的初始爆破和更稳定的释放曲线。 因此,通过调节乳酸/羟基乙酸比、分子质量和端基团可以改变PLGA聚合物的吸水率,进而影响聚合物水解和侵蚀,从而控制药物释放速度[22]。但是微球的药物包封率和释放曲线不仅取决于聚合物自身的性质,还与所负载药物的理化性质、溶解有机相载体的溶剂以及制备方法有关[12]。一般来说,亲水性化合物比亲脂性化合物更难负载,且亲水性物质的释放速度更快,因为亲脂性物质会阻碍水分子进入微球,从而降低聚合物的降解速度。与单纯PBS相比,在模拟酶存在的条件下,PLGA的降解速率进一步加快。因此,当研究者们选择PLGA作为载体材料时,除了PLGA自身的性质之外,研究者们还应该考虑负载药物的性质、溶剂对聚合物影响及应用的生理环境等其他方面。下面介绍常见的PLGA微球的制备方法,以进一步探究不同方法制备的微球在骨组织工程中的应用。"


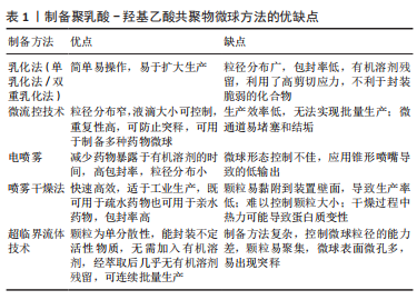
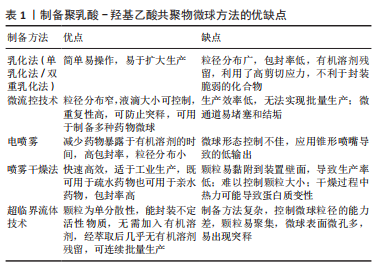
2.2 PLGA的制备方法 2.2.1 乳化法(单乳化法/双重乳化法) 乳化法是制备PLGA微球最常用的方法,可分为单乳化方法(O/W)和双重乳化方法(W1/O/W2、S/O/W和W/O/O)。根据包埋药物分子的不同理化性质,可以选择不同的制备方法。PLGA聚合物和疏水性药物可溶于有机溶剂中,如氯仿或二氯甲烷,然后加入含有表面活性剂如聚乙烯醇的水相,用高速均质器或超声探头将有机相在大量的水相中乳化,得到O/W乳液[23]。然后,有机溶剂可以在温和的氮气流中,或在真空状态进行搅拌与挥发,液滴收缩并凝固成聚合物颗粒,得到的颗粒通过离心、洗涤以去除剩余的表面活性剂,然后冻干以便进一步储存[24]。由于蛋白质和核酸的亲水性,使其在聚合物固化前就会扩散到水相中,因此单乳化法不适合制备负载生物大分子的PLGA颗粒。双重乳化法可用于进一步包封亲水性药物,将含有亲水性药物的水溶液加入到溶有PLGA的有机相中,在剧烈搅拌下得到W/O乳液,将该乳状液加入含有高剪切能表面活性剂的第二水溶液,形成W/O/W乳状液。然后,再通过溶剂蒸发得到凝固的颗粒[25]。因此乳化法既可用于疏水药物的包埋,也可用于亲水药物的包埋。此外,它还可以通过调节搅拌速度和进料速度来制备从纳米到微米的颗粒[16]。 虽然乳化法简单、易操作,但其制备的微球存在粒径分布广和包封率低等问题[22],此外溶剂蒸发需要加热或真空而且稳定剂难以完全清除[26]。两种乳化法都利用了高剪切应力,这不利于封装蛋白质。近年来,膜挤压技术被用来制备乳化后粒径分布较窄的颗粒,常使用聚碳酸酯薄膜在高压下进行薄膜挤出[24]。 2.2.2 微流控技术 微流控技术是一种生产高度可控、均匀微球的新型策略[27]。液滴形成的整个过程是在含有微米级通道的微流控装置中进行的,不相容的连续相(如药物-聚合物溶液)和含表面活性剂的水相在不同的独立入口处被注入装置,由于高剪切力,在不同相的通道交叉处可以形成颗粒。微流控装置有不同形状的通道,其中制备微球最常用的是T形通道、同向通道和聚流通道[28-30]。T形通道的装置制备工艺简单,适用于制备高单分散性的微滴。在微流控装置中,液滴的化学成分可以根据不同相的组成而改变,液滴的大小可以根据不同相的流速来控制,使得该技术非常适合于制备高度复杂的药物载体[31]。而且可以设计具有特定形状微通道的微流控装置,以满足不同微球的功能和结构需求,从而实现微球的定制化[32]。 微流控法制备的PLGA微球具有粒径分布窄、重复性高、可防止初始释放等优点。此外,该微流控系统常用于制备各种表面修饰的PLGA微球。然而,它的局限性包括生产效率低、无法实现批量生产以及微通道易堵塞和结垢等相关问题[31]。因此,有研究提出将微流控装置并行化,设计出同时包含多个液滴发生器的芯片,这种具有多层几何结构的液滴发生器可以极大地简化液滴的生产过程,提高微球生产效率[33]。 2.2.3 电喷雾 电喷雾是将含有药物的PLGA首先溶解在充分导电的溶剂中,装入与针头连接的注射器中,然后在针和收集器之间施加高压,在针尖产生PLGA溶液气溶胶。在PLGA液滴飞向收集器的过程中,溶剂蒸发,产生的PLGA药物微粒被收集。电喷雾产生的PLGA颗粒带有很高的电荷,这些电荷产生的静电力超过溶液的表面张力,阻止了它们的凝聚,促进颗粒的自分散,从而产生尺寸分布均匀的液滴[34]。 与其他技术相比,电喷雾的优点是减少了药物暴露于有机溶剂的时间,并且具有高包封率[35]。电喷雾颗粒的尺寸可以非常小,从几微米到几纳米,并且可以通过改变PLGA溶液的电压和流量在一定程度上进行控制,微球粒径随着聚合物浓度和喷雾距离的增加而有所减小,而且具有较小的粒径分布[36]。缺点在于锥形喷嘴导致微球的形态控制不佳,而且微球的产量较低。 2.2.4 喷雾干燥 喷雾干燥是一种将药物和聚合物溶液、悬浮液或乳状液雾化并注入热空气中的技术。主要是将液体进料喷洒到热干燥介质(空气、惰性气体如氮气)中,将其转化为颗粒的过程[37]。喷雾干燥法制备条件温和、工艺参数简单、快速、方便,因此适合于工业放大。此方法既可用于疏水药物也可用于亲水药物的包埋,药物的类型(疏水性或亲水性)决定了在该过程中使用的溶剂的选择。所用溶剂的性质、溶剂蒸发温度和进料速度影响最终产品的形貌。研究表明,这种方法可以将大多数药物/蛋白质包裹成微粒,而不会显著降低它们的生物活性。但这项技术的主要缺点是微粒容易附着在喷雾干燥器的内壁上,导致生产率降低[38],这可以通过防粘剂来改善;此外,颗粒大小难以控制,干燥过程可能会导致蛋白质变性。随着制药工业对精确控制固体粒子性能的要求日益提高,其应用正在转向最近文献中的“粒子工程”,因为它能够通过配方和工艺参数的组合,以独特的高精度操纵单个粒子的属性[39-40]。 2.2.5 超临界流体法 超临界流体法也被应用于制备微球。在使用的各种方法中,超临界二氧化碳能替代传统的有机溶剂,而且工艺条件温和、环境友好,因此受到特别关注。超临界CO2具有相对较低的临界温度(31.1 ℃)和临界压力(7.4 MPa),具有溶解某些聚合物的独特能力[41]。在超临界流体法中,聚合物溶液(溶解在超临界CO2中)通过喷嘴减压进入低压环境,快速减压形成颗粒。压力、喷嘴直径、溶液浓度和温度是影响最终颗粒性质的关键工艺参数。目前有各种各样的技术涉及超临界流体,包括超临界溶液的快速膨胀、气体反溶剂过程、超临界反溶剂过程以及超临界流体的溶液增强分散[42]。选择最合适的加工技术很大程度上取决于超临界CO2与活性成分、聚合物包衣材料和合适的溶剂的相互作用[43]。超临界CO2流体萃取方法在制备微球的过程中无需加入有机溶剂,或经萃取后几乎无有机溶剂残留,可连续批量生产,但此法控制微球粒径的能力差,且微球表面微孔多,易出现突释现象。而且超临界流体技术的操作步骤和变量条件较多,不太适合大众研究,多用于药物的纯化提炼,一定程度上限制了其在制作微球中的应用。 制备PLGA微球方法的示意图见图3,优缺点总结见表1。"

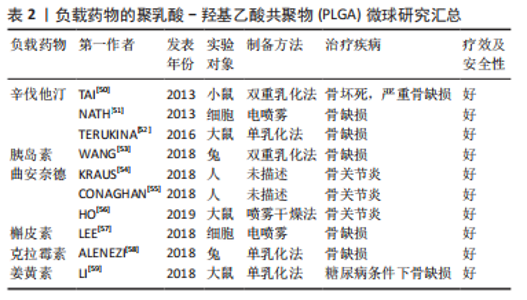
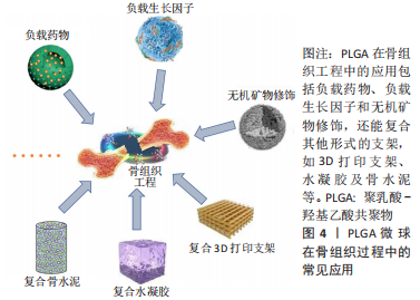
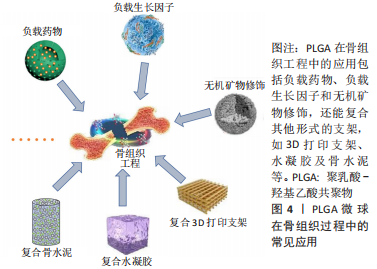
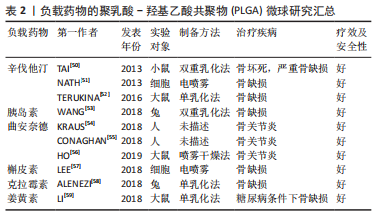
2.3.1 负载生长因子和药物 具有促进细胞增殖、分化、血管生成和成骨作用的生长因子和药物通常在PLGA微球的制备过程中被负载到微球内部或通过后处理吸附到微球的表面。常用的细胞因子包括转化生长因子β、胰岛素样生长因子和血管内皮生长因子等,骨形态发生蛋白2作为转化生长因子β超家族成员之一,能有效促进骨再生。因此,有研究通过微流控技术制备了负载有血管内皮细胞生长因子的PLGA微球,然后在微球表面用聚(3,4-二羟基苯乙胺)固定骨形态发生蛋白2,以用于促进血管生成及骨再生[44]。也有研究制备了纳米氢氧化铁包覆的PLGA微球,将纳米颗粒均匀地固定在PLGA微球表面,然后将这些微球烧结成支架,将骨形态发生蛋白2通过多巴胺包被固定在支架表面从而诱导成骨分化[45],结果表明,在支架上培养的骨髓间充质干细胞具有良好的增殖、成骨分化和迁移能力。除了表面涂层外,生长因子还可以通过其他生物活性中间体负载到PLGA微球中,以获得更好的缓释效果。然而,挑战在于将亲水性蛋白质分子插入疏水聚合物微球的核心。为了解决这个障碍,有研究用改进的双乳化法,制备了PLGA-介孔硅基多级载体复合微球,结果表明,复合微球对骨形态发生蛋白2有较长时间的持续释放,而且复合微球对细胞无毒性,所释放的骨形态发生蛋白2生物活性保持不变[46]。也有研究以大豆卵磷脂为载体,通过两步法制备了负载骨形态发生蛋白2的PLGA/大豆卵磷脂微球,实验表明,与单纯PLGA微球相比,该微球具有更高的包封率和可控的释放行为[47]。针对生长因子的负载,主动自封装是一种新发展的后负载方法,它基于载负电多糖(捕捉剂)的微孔PLGA微球对带正电的蛋白质的吸附。研究人员将胰岛素样生长因子、血管内皮生长因子和成纤维细胞生长因子负载到以高分子葡聚糖硫酸盐为捕捉剂的PLGA微球中。发现在胰岛素样生长因子快速释放的同时,血管内皮生长因子和成纤维细胞生长因子以生物活性形式持续释放4周,具有协同血管生成的作用。因此,主动自封装可以使生物活性生长因子的持续释放,对生物材料植入物的血管化具有很大的吸引力[48]。生长因子和趋化因子的使用,实现了特殊的生物功能要求,在骨和软骨组织的修复中发挥了重要的作用。然而,缺点在于它们在微球制备过程中的失活是不容忽视的,而且植入体内后代谢周期可能会存在不良反应和异位成骨的风险[49]。 除了生物活性因子,具有骨促进或血管形成性质的药物也被负载到PLGA微球中用于骨再生研究。辛伐他汀是一种还原酶抑制剂,已被证明能够治疗骨坏死和促进血管生成[50]。NATH等[51]用电喷雾技术制备了负载辛伐他汀的PLGA微球,辛伐他汀的包封率在90%以上。此外,TERUKINA等[52]评估了不同直径PLGA微球释放辛伐他汀对体外和体内骨再生的影响,结果表明辛伐他汀在微球中可持续释放1个月,而在纳米球中只能持续释放1周,载药微球更有利于细胞增殖、分化和骨矿化。WANG等[53]采用双乳化法制备了粒径均匀的负载胰岛素的PLGA微球,用于种植体周围骨形成。关节内应用皮质类固醇可缓解骨关节炎疼痛,但全身快速吸收限制了疗效。FX006是一种基于微球的新型缓释曲安奈德制剂,与标准曲安奈德晶体混悬剂相比,它延长了曲安奈德在关节的停留时间,减少了全身暴露[54]。有研究向膝关节骨性关节炎患者的关节内注射FX006,结果表明膝关节骨性关节炎的疼痛持续性的减轻,且观察到疼痛强度减少的幅度和持续时间都优于传统的给药方式[55]。HO等[56]用喷雾干燥法制备了负载曲安奈德微晶的PLGA微球,药物微晶均匀包埋在微球中,延长药物在关节中的滞留时间,有望在减少给药频率的情况下有益于骨关节炎患者。有研究采用电喷雾技术制备了载槲皮素PLGA微球,结果表明槲皮素生物降解微球释放时间延长,骨矿化度增加[57],有利于成骨。载有其他药物的PLGA微球,如克拉霉素和姜黄素,也被广泛用于骨形成[58-59]。PLGA微球可注射、微创、缓释药物的优点,使得载药微球可能成为多种疾病的治疗选择。在目前,生物活性因子的不稳定性和副反应限制了它们在骨再生中的应用[60]。因此,在PLGA骨修复材料中引入小分子药物等成本低、生化稳定性好的成分可能更容易生产和广泛应用。 文章总结了负载药物的PLGA微球研究成果,见表2。"
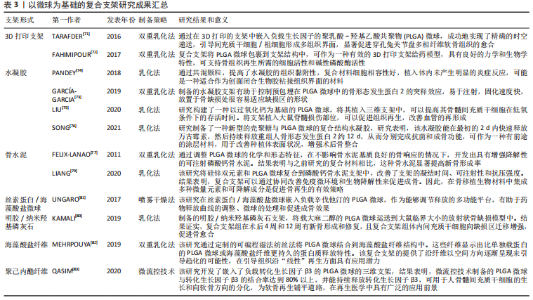
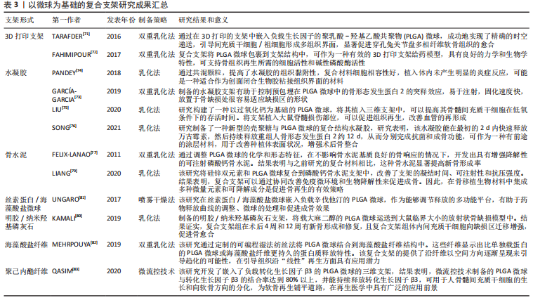
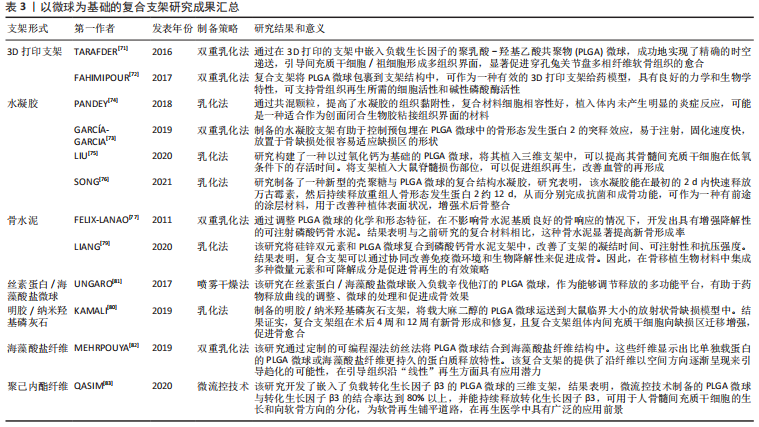
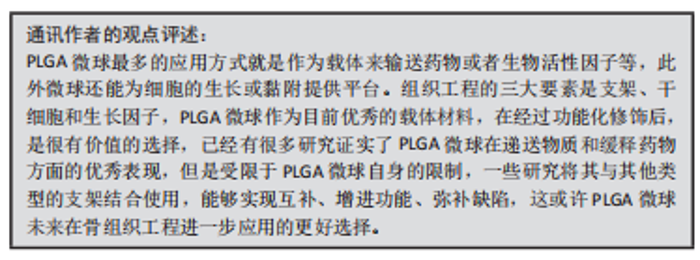
2.3.2 功能化修饰 为了改善骨传导性、中和PLGA微球降解造成的酸性环境,无机矿物通常被用来使PLGA微球具有生物矿化和成骨作用,聚合物/生物陶瓷复合微球已被用于骨再生,达到解决聚合物的力学性能及生物活性较弱的问题,并能够通过微创注射填充不规则的骨缺损。磷酸三钙是天然骨组织中的一种无机成分,基于磷酸钙的制剂表现出良好的骨传导性和生物相容性[61],将磷酸三钙添加到PLGA中,可减少溶解时的pH值下降,并缓冲酸性降解产物,在不同研究中已经证明了成骨特性、相对稳定性以及与宿主骨组织的强键形成[62]。在以往的研究中,通过在PLGA/磷酸三钙微球中加入骨形态发生蛋白2和血管内皮生长因子等活性成分,研究人员取得了满意的骨修复效果[63]。羟基磷灰石也是人体骨组织的组成成分,羟基磷灰石具有骨诱导性,对骨组织的形成有促进作用,但其抗疲劳性能低、降解性差,不宜直接作为人工骨组织材料[64]。有研究采用双乳化法制备了负载磷脂酰化阿托伐他汀的PLGA微球,然后用羟基磷灰石包被,将脂肪源性间充质干细胞接种于微球上培养,结果表明,与其余组别相比实验组的成骨分化最强[65]。综上,无机矿物的修饰能弥补PLGA微球的不足,为其在骨组织工程中的应用提供新的思路。 细胞疗法是一种新兴的组织再生方法,通过调控细胞的分泌和活性可以控制细胞的微环境和功能,比如调节干细胞表型来介导骨组织再生的策略是可行和有前景的。ANKRUM等[66]提供了一种基于PLGA微球的平台来成功控制间充质干细胞表型的方案。CURRAN等[67]利用等离子体处理制备了表面含有功能性化学基团的PLGA微球,可以刺激间充质干细胞向特定谱系分化。他们证实,这种方案可以在没有外源物质补充的情况下诱导间充质干细胞产生所需的生长因子。在另一项研究中,外周血来源的间充质干细胞和内皮集落形成细胞被用于使纤维蛋白凝胶/PLGA微球具有预血管化功能[68],结果表明载有间充质干细胞和内皮集落形成细胞的微球倾向于形成毛细血管状结构,穿透微球之间的间隙并在体内与天然毛细血管融合,这项研究为有效的血管化组织再生提供了策略。此外,LIU等[69]设计了一种多生物递送载体,其中介孔硅、PLGA微球和聚乳酸纳米纤维海绵状微球可以通过释放白细胞介素2、转化生长因子β和miR-10a来局部募集T细胞,并触发其分化为调节性T细胞。结果表明,这种递送系统可以聚集调节T细胞,介导免疫稳态,从而治疗牙周骨质流失。随着对骨组织再生机制理解的深入,基于PLGA微球的细胞调控平台有望进一步发展,尽早干预疾病进程。 2.3.3 微球为基础的复合支架 PLGA微球作为一种理想的给药载体,经常与其他形式的支架结合使用,以提高药物的持续或顺序释放能力,调整降解行为[70]。由于PLGA的快速降解特性,将PLGA微球负载到支架中也可以作为致孔剂,从而提高支架的整体降解率,促进骨整合和血管化。目前,微球通常被植入3D打印支架、水凝胶、骨水泥或其他形式的支架中,以提高支架的整体性能。 TARAFDER等[71]开发了一种具有时空传递系统的复合支架,用于复杂的非均匀组织再生。通过将生长因子封装到PLGA微球中,并将其嵌入3D打印支架中,实现了生长因子在所需位置的精准输送,并具有持续的释放行为。FAHIMIPOUR等[72]报道了一种基于3D磷酸三钙的支架,其中含有负载血管内皮生长因子的PLGA微球。实验表明,释放的血管内皮生长因子能够满足组织再生早期的血管化要求,并能显著提高碱性磷酸酶活性,提示该复合支架是一种潜在的颅面组织再生材料。 在另一项研究中,GARCíA-GARCIA等[73]制备了一种包埋含骨形态发生蛋白2的PLGA微球的复合水凝胶,用于骨质疏松骨缺损的骨再生,PLGA微球的加入可以提高水凝胶的机械强度和与组织界面的黏附性。PANDEY等[74]通过对N-羟基琥珀酰亚胺修饰的PLGA微球进行改性,开发了一种贻贝启发的黏附性水凝胶。PLGA微球的存在显著提高了组织黏附强度,并引起了最小的炎症反应。此外有研究将过氧化钙分散在PLGA中形成微球,然后将这些微球放入一种温度敏感的自组装肽水凝胶中,可以在局部维持氧气的释放。从而提高骨髓间充质干细胞在体外缺氧条件下的存活能力,并能促进脊髓损伤大鼠的神经再生,减少空洞形成[75]。有研究还制备了由壳聚糖水凝胶和闭孔PLGA微球组成的新型复合结构水凝胶[76],结果表明这种材料可以在最初2 d快速释放万古霉素,然后持续释放重组人骨形态发生蛋白2约12 d,分别完成抗菌和成骨功能,从而增强术后的骨整合。综上所述,基于功能性PLGA微球的支架材料的发展可能会促进种植体与宿主组织之间的界面黏附和骨结合。 磷酸钙骨水泥以其良好的注射性、生物活性和成骨性被广泛应用于骨缺损修复,然而,缺乏可降解性抑制了新形成的骨渗透到支架中。有研究报告了一种含有改性PLGA微球的快速降解磷酸钙骨水泥[77],研究结果发现,酸性端基的PLGA微球植入兔股骨髁6周后,显示出相当大的孔隙率和骨长入。因此,PLGA微球作为一种致孔剂,可以在不影响骨传导性的前提下,通过酸性降解产物有效地溶解磷酸钙骨水泥,提高其降解率[78]。最近,有研究报道了负载硅/锌的PLGA/磷酸钙骨水泥支架的骨再生协同作用[79]。结果表明,PLGA微球和硅/锌双元素的加入提高了磷酸钙骨水泥支架的凝结时间、可注性和抗压强度。有趣的是,含有硅/锌双元素的PLGA/磷酸钙骨水泥促进了小鼠单核巨噬细胞向M2表型的极化,并促进了抗炎基因的表达;组织学染色结果显示,12周后新形成的骨渗透到PLGA微球中,24周后新骨几乎完全占据了PLGA微球降解形成的空间。上述工作表明,微球与磷酸钙骨水泥的结合确实是提高支架整体性能的一种很好的策略。 除了与3D打印支架、水凝胶和骨水泥结合使用外,PLGA微球还经常作为药物载体与传统的支架或纺丝结合用于骨组织工程。有研究制备了一种明胶/纳米羟基磷灰石支架,将负载大麻二酚的PLGA微球与支架结合,用于大鼠桡骨缺损模型,所制备的支架具有良好的生物相容性和成骨诱导活性[80]。有研究提出在丝素蛋白/海藻酸盐微球中嵌入负载辛伐他汀的PLGA微球,调节药物的释放,从而实现成骨作用[81]。丝素蛋白/海藻酸盐微球可以作为一个合适的平台来包埋负载辛伐他汀的PLGA微球,以便于药物释放曲线的调整、微球的处理和促进成骨作用。如果开发得当,多功能杂化微球可能特别适合于制备具有长期生物活性的支架。另一项研究中,提出了一种制备两相海藻酸盐/PLGA纤维蛋白输送系统的技术[82],FITC-BSA(一种模型蛋白质)被包裹在PLGA微球中,然后通过设计的可编程湿法纺丝方法并入海藻酸盐纤维结构中。该研究证明了海藻酸盐纤维的生产是可靠的,而且嵌入蛋白的释放受到控制。也有研究以乳化法制备PLGA微球后将其成功地与转化生长因子β3偶联并嵌入到聚己内酯纤维中,用于促进骨髓间充质干细胞增殖和软骨分化[83]。通过与不同类型支架的复合,可以发现PLGA微球可以被广泛应用于多种生物材料,PLGA微球自身的可降解性、缓释药物等优点被充分利用,提高了复合支架的整体性能。而其他材料也能弥补PLGA微球的缺陷,比如碱性物质的加入中和了PLGA微球降解的酸性产物,骨水泥的使用增加了支架的力学性能,并提供了成骨所需物质,水凝胶的使用增加支架与组织或细胞之间的黏附与结合。 综上,与单纯应用一种材料相比,或许复合支架能更好地发挥出不同材料的优点,互相弥补不足,以达到预期效果。然而,如何选择合适的材料,如何选择合适的配比,还需要对骨组织修复机制有更深的了解。 文章总结了以微球为基础的复合支架研究成果,见表3。"
| [1] BLASI P. Poly (lactic acid)/poly (lactic-co-glycolic acid)-based microparticles: an overview. J Pharm Investig. 2019;49(4):337-346. [2] YUAN Z, WEI P, HUANG Y, et al. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019;85: 294-309. [3] ZHONG H, CHAN G, HU Y, et al. A Comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10(4):263. [4] DANHIER F, ANSORENA E, SILVA JM, et al. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505-522. [5] SU Y, ZHANG B, SUN R, et al. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv. 2021;28(1):1397-418. [6] ARMIENTO AR, HATT LP, SANCHEZ ROSENBERG G, et al. Functional biomaterials for bone regeneration: a lesson in complex biology. Adv Funct Mater. 2020. doi:10.1002/adfm.201909874. [7] ZHAO D, ZHU T, LI J, et al. Poly (lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact Mater. 2021;6(2):346-360. [8] PARK CW, LEE HJ, OH DW, et al. Preparation and in vitro/in vivo evaluation of PLGA microspheres containing norquetiapine for long-acting injection. Drug Des Devel Ther. 2018;12:711-719. [9] LI X, WEI Y, WEN K, et al. Novel insights on the encapsulation mechanism of PLGA terminal groups on ropivacaine. Eur J Pharm Biopharm. 2021;160:143-151. [10] ESSA D, KONDIAH PPD, CHOONARA YE, et al. The Design of Poly (lactide-co-glycolide) nanocarriers for medical applications. Front Bioeng Biotechnol. 2020;8:48. [11] KIM KT, LEE J Y, KIM DD, et al. Recent progress in the development of Poly (lactic-co-glycolic acid)-based nanostructures for cancer imaging and therapy. Pharmaceutics. 2019;11(6):280. [12] KOERNER J, HORVATH D, GROETTRUP M. Harnessing dendritic cells for Poly (D,L-lactide-co-glycolide) microspheres (PLGA MS)-mediated anti-tumor therapy. Front Immunol. 2019;10:707. [13] HSU MY, FENG CH, LIU YW, et al. An orthogonal model to study the effect of electrospraying parameters on the morphology of poly (d,l)-lactide-co-glycolide (PLGA) particles. Appl Sci-Basel. 2019. doi:10.3390/app9061077. [14] SAINI V, JAIN V, SUDHEESH M S, et al. Comparison of humoral and cell-mediated immune responses to cationic PLGA microspheres containing recombinant hepatitis B antigen. Int J Pharm. 2011;408(1-2):50-57. [15] SILVA AL, ROSALIA RA, SAZAK A, et al. Optimization of encapsulation of a synthetic long peptide in PLGA nanoparticles: low-burst release is crucial for efficient CD8(+) T cell activation. Eur J Pharm Biopharm. 2013;83(3):338-345. [16] ALLAHYARI M, MOHIT E. Peptide/protein vaccine delivery system based on PLGA particles. Hum Vaccin Immunother. 2016;12(3):806-828. [17] HAN FY, THURECHT KJ, WHITTAKER AK, et al. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front Pharmacol. 2016;7:185. [18] KUMARI A, YADAV SK, YADAV SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1-18. [19] WANG T, XUE P, WANG A, et al. Pore change during degradation of octreotide acetate-loaded PLGA microspheres: the effect of polymer blends. Eur J Pharm Sci. 2019;138:104990. [20] WAN F, YANG M. Design of PLGA-based depot delivery systems for biopharmaceuticals prepared by spray drying. Int J Pharm. 2016;498(1-2): 82-95. [21] WANG J, HELDER L, SHAO J, et al. Encapsulation and release of doxycycline from electrospray-generated PLGA microspheres: effect of polymer end groups. Int J Pharm. 2019;564:1-9. [22] ZHANG C, YANG L, WAN F, et al. Quality by design thinking in the development of long-acting injectable PLGA/PLA-based microspheres for peptide and protein drug delivery. Int J Pharm. 2020;585:119441. [23] MUNDARGI RC, BABU VR, RANGASWAMY V, et al. Nano/micro technologies for delivering macromolecular therapeutics using poly (D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125(3):193-209. [24] SWIDER E, KOSHKINA O, TEL J, et al. Customizing poly (lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018;73:38-51. [25] MIR M, AHMED N, REHMAN A U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159: 217-231. [26] REZVANTALAB S, DRUDE N I, MORAVEJI M K, et al. PLGA-based nanoparticles in cancer treatment. Front Pharmacol. 2018;9:1260. doi:10.3389/fphar. 2018.01260 [27] LI G, YAO L, LI J, et al. Preparation of poly(lactide-co-glycolide) microspheres and evaluation of pharmacokinetics and tissue distribution of BDMC-PLGA-MS in rats. Asian J Pharm Sci. 2018;13(1):82-90. [28] BRZEZIŃSKI M, SOCKA M, KOST B. Microfluidics for producing polylactide nanoparticles and microparticles and their drug delivery application. Polym Int. 2019;68(6):997-1014. [29] ZOU Q, HOU F, WANG H, et al. Microfluidic one-step preparation of alginate microspheres encapsulated with in situ-formed bismuth sulfide nanoparticles and their photothermal effect. Eur Polym J. 2019;115:282-289. [30] HE T, WANG W, CHEN B, et al. 5-Fluorouracil monodispersed chitosan microspheres: Microfluidic chip fabrication with crosslinking, characterization, drug release and anticancer activity. Carbohydr Polym. 2020;236:116094. [31] PENG H-Y, WANG W, XIE R, et al. Mesoscale regulation of droplet templates to tailor microparticle structures and functions. Particuology. 2020;48:74-87. [32] ZENG W, GUO P, JIANG P, et al. Combination of microfluidic chip and electrostatic atomization for the preparation of drug-loaded core-shell nanoparticles. Nanotechnology. 2020;31(14):145301. [33] NAWAR S, STOLAROFF JK, YE C, et al. Parallelizable microfluidic dropmakers with multilayer geometry for the generation of double emulsions. Lab Chip. 2020;20(1): 147-154. [34] YAO ZC, JIN LJ, AHMAD Z, et al. Ganoderma lucidum polysaccharide loaded sodium alginate micro-particles prepared via electrospraying in controlled deposition environments. Int J Pharm. 2017;524(1-2):148-158. [35] DING L, LEE T, WANG CH. Fabrication of monodispersed Taxol-loaded particles using electrohydrodynamic atomization. J Control Release. 2005; 102(2):395-413. [36] MORAIS AIS, VIEIRA EG, AFEWERKI S, et al. Fabrication of polymeric microparticles by electrospray: the impact of experimental parameters. J Funct Biomater. 2020; 11(1):4. [37] WEI H, LI W, CHEN H, et al. Simultaneous Diels-Alder click reaction and starch hydrogel microsphere production via spray drying. Carbohydr Polym. 2020;241:116351. [38] DING D, ZHU Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater Sci Eng C Mater Biol Appl. 2018;92:1041-1060. [39] MEEUS J, LENAERTS M, SCURR DJ, et al. The influence of spray-drying parameters on phase behavior, drug distribution, and in vitro release of injectable microspheres for sustained release. J Pharm Sci. 2015;104(4):1451-1460. [40] MEEUS J, SCURR DJ, APPELTANS B, et al. Influence of formulation composition and process on the characteristics and in vitro release from PLGA-based sustained release injectables. Eur J Pharm Biopharm. 2015;90:22-29. [41] TEEKAMP N, DUQUE LF, FRIJLINK HW, et al. Production methods and stabilization strategies for polymer-based nanoparticles and microparticles for parenteral delivery of peptides and proteins. Expert Opin Drug Deliv. 2015;2(8):1311-1331. [42] GANGAPURWALA G, VOLLRATH A, DE SAN LUIS A, et al. PLA/PLGA-based drug delivery systems produced with supercritical CO2-a green future for particle formulation? Pharmaceutics. 2020;12(11):1118. [43] SOH SH, LEE LY. Microencapsulation and nanoencapsulation using supercritical fluid (SCF) techniques. Pharmaceutics. 2019;11(1):21. [44] DASHTIMOGHADAM E, FAHIMIPOUR F, TONGAS N, et al. Microfluidic fabrication of microcarriers with sequential delivery of VEGF and BMP-2 for bone regeneration. Sci Rep. 2020;10(1):11764. [45] ZHANG F, LI Q, LIN Z, et al. Engineered Fe(OH)3 nanoparticle-coated and rhBMP-2-releasing PLGA microsphere scaffolds for promoting bone regeneration by facilitating cell homing and osteogenic differentiation. J Mater Chem B. 2018;6(18):2831-2842. [46] MINARDI S, FERNANDEZ-MOURE JS, FAN D, et al. Biocompatible PLGA-mesoporous silicon microspheres for the controlled release of BMP-2 for bone augmentation. Pharmaceutics. 2020;12(2):118. [47] WEI D, QIAO R, DAO J, et al. Soybean lecithin-mediated nanoporous PLGA microspheres with highly entrapped and controlled released BMP-2 as a stem cell platform. Small. 2018;14(22):e1800063. [48] SCHEINER KC, MAAS-BAKKER RF, VAN STEENBERGEN MJ, et al. Post-loading of proangiogenic growth factors in PLGA microspheres. Eur J Pharm Biopharm. 2021; 158:1-10. [49] CUI L, ZHANG J, ZOU J, et al. Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials. 2020;230:119617. [50] TAI I C, FU YC, WANG CK, et al. Local delivery of controlled-release simvastatin/PLGA/HAp microspheres enhances bone repair. Int J Nanomedicine. 2013;8:3895-904. [51] NATH SD, SON S, SADIASA A, et al. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int J Pharm. 2013;443(1-2):87-94. [52] TERUKINA T, NAITO Y, TAGAMI T, et al. The effect of the release behavior of simvastatin from different PLGA particles on bone regeneration in vitro and in vivo: comparison of simvastatin-loaded PLGA microspheres and nanospheres. J Drug Deliv Sci Tec. 2016;33:136-142. [53] WANG X, WANG L, QI F, et al. The effect of a single injection of uniform-sized insulin-loaded PLGA microspheres on peri-implant bone formation. RSC Adv. 2018;8(70):40417-40425. [54] KRAUS VB, CONAGHAN PG, AAZAMI HA, et al. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA). Osteoarthritis Cartilage. 2018;26(1):34-42. [55] CONAGHAN PG, HUNTER DJ, COHEN SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100(8):666-677. [56] HO MJ, JEONG HT, IM SH, et al. Design and in vivo pharmacokinetic evaluation of triamcinolone acetonide microcrystals-loaded PLGA microsphere for increased drug retention in knees after intra-articular injection. Pharmaceutics. 2019;11(8):419. [57] LEE H, NGUYEN TT, KIM M, et al. The effects of biodegradable poly(lactic-co-glycolic acid)-based microspheres loaded with quercetin on stemness, viability and osteogenic differentiation potential of stem cell spheroids. J Periodontal Res. 2018;53(5):801-815. [58] ALENEZI A, NAITO Y, TERUKINA T, et al. Controlled release of clarithromycin from PLGA microspheres enhances bone regeneration in rabbit calvaria defects. J Biomed Mater Res B Appl Biomater. 2018;106(1):201-208. [59] LI Y, ZHANG ZZ. Sustained curcumin release from PLGA microspheres improves bone formation under diabetic conditions by inhibiting the reactive oxygen species production. Drug Des Devel Ther. 2018;12:1453-1466. [60] JIN S, SUN F, ZOU Q, et al. Fish collagen and hydroxyapatite reinforced Poly (lactide- co-glycolide) fibrous membrane for guided bone regeneration. Biomacromolecules. 2019;20(5):2058-2067. [61] JAZAYERI H E, TAHRIRI M, RAZAVI M, et al. A current overview of materials and strategies for potential use in maxillofacial tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):913-929. [62] PARK KD, JUNG YS, LEE KK, et al. Behavior of osteoblast-like cells on a beta-tricalcium phosphate synthetic scaffold coated with calcium phosphate and magnesium. J Craniofac Surg. 2016;27(4):898-903. [63] ZHANG HX, ZHANG XP, XIAO GY, et al. In vitro and in vivo evaluation of calcium phosphate composite scaffolds containing BMP-VEGF loaded PLGA microspheres for the treatment of avascular necrosis of the femoral head. Mater Sci Eng C Mater Biol Appl. 2016;60:298-307. [64] DILEEPKUMAR VG, SRIDHAR MS, ARAMWIT P, et al. A review on the synthesis and properties of hydroxyapatite for biomedical applications. J Biomater Sci Polym Ed. 2022;33(2):229-261. [65] SHOKROLAHI F, KHODABAKHSHI K, SHOKROLLAHI P, et al. Atorvastatin loaded PLGA microspheres: preparation, HAp coating, drug release and effect on osteogenic differentiation of ADMSCs. Int J Pharm. 2019;565:95-107. [66] ANKRUM JA, MIRANDA OR, NG KS, et al. Engineering cells with intracellular agent-loaded microparticles to control cell phenotype. Nat Protoc. 2014;9(2):233-245. [67] CURRAN JM, FAWCETT S, HAMILTON L, et al. The osteogenic response of mesenchymal stem cells to an injectable PLGA bone regeneration system. Biomaterials. 2013;34(37):9352-9364. [68] LIU J, CHEN G, XU H, et al. Pre-vascularization in fibrin Gel/PLGA microsphere scaffolds designed for bone regeneration. Npg Asia Mater. 2018;10(8):827-839. [69] LIU Z, CHEN X, ZHANG Z, et al. Nanofibrous spongy microspheres to distinctly release mirna and growth factors to enrich regulatory t cells and rescue periodontal bone loss. ACS Nano. 2018;12(10):9785-9799. [70] MINARDI S, CORRADETTI B, TARABALLI F, et al. Biomimetic concealing of PLGA microspheres in a 3D scaffold to prevent macrophage uptake. Small. 2016;12(11): 1479-1488. [71] TARAFDER S, KOCH A, JUN Y, et al. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016; 8(2):025003. [72] FAHIMIPOUR F, RASOULIANBOROUJENI M, DASHTIMOGHADAM E, et al. 3D printed TCP-based scaffold incorporating VEGF-loaded PLGA microspheres for craniofacial tissue engineering. Dent Mater. 2017;33(11):1205-1216. [73] GARCIA-GARCIA P, REYES R, SEGREDO-MORALES E, et al. PLGA-BMP-2 and PLA-17beta-estradiol microspheres reinforcing a composite hydrogel for bone regeneration in osteoporosis. Pharmaceutics. 2019;11(12):648. [74] PANDEY N, HAKAMIVALA A, XU C, et al. Biodegradable nanoparticles enhanced adhesiveness of mussel-like hydrogels at tissue interface. Adv Healthc Mater. 2018; 7(7):e1701069. [75] LIU L, WAN J, DAI M, et al. Effects of oxygen generating scaffolds on cell survival and functional recovery following acute spinal cord injury in rats. J Mater Sci Mater Med. 2020;31(12):115. [76] SONG W, XIAO Y. Sequential drug delivery of vancomycin and rhBMP-2 via pore-closed PLGA microparticles embedded photo-crosslinked chitosan hydrogel for enhanced osteointegration. Int J Biol Macromol. 2021;182:612-625. [77] FELIX LANAO RP, LEEUWENBURGH SC, WOLKE JG, et al. Bone response to fast-degrading, injectable calcium phosphate cements containing PLGA microparticles. Biomaterials. 2011;32(34):8839-8847. [78] FELIX LANAO RP, LEEUWENBURGH SC, WOLKE JG, et al. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater. 2011;7(9):3459-3468. [79] LIANG W, GAO M, LOU J, et al. Integrating silicon/zinc dual elements with PLGA microspheres in calcium phosphate cement scaffolds synergistically enhances bone regeneration. J Mater Chem B. 2020;8(15):3038-3049. [80] KAMALI A, ORYAN A, HOSSEINI S, et al. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater Sci Eng C Mater Biol Appl. 2019;101:64-75. [81] UNGARO F, CATANZANO O, D’ANGELO I, et al. Microparticle-embedded fibroin/alginate beads for prolonged local release of simvastatin hydroxyacid to mesenchymal stem cells. Carbohydr Polym. 2017;175:645-653. [82] MEHRPOUYA F, YUE Z, ROMEOT, et al. A simple technique for development of fibres with programmable microsphere concentration gradients for local protein delivery. J Mater Chem B. 2019;7(4):556-565. [83] QASIM M, LE N XT, NGUYEN TPT, et al. Nanohybrid biodegradable scaffolds for TGF-beta3 release for the chondrogenic differentiation of human mesenchymal stem cells. Int J Pharm. 2020;581:119248. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [3] | De Ji, Suo Langda, Wei Yuchen, Wang Bin, Awangcuoji, Renqingcuomu, Cui Jiuzeng, Zhang Lei, Ba Gui. Comprehensive analysis of genes related to endometrial receptivity and alternative splicing events in northwest Tibetan cashmere goats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1429-1436. |
| [4] | Peng Hongcheng, Peng Guoxuan, Lei Anyi, Lin Yuan, Sun Hong, Ning Xu, Shang Xianwen, Deng Jin, Huang Mingzhi . Role and mechanism of platelet-derived growth factor BB in repair of growth plate injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1497-1503. |
| [5] | Chen Yuning, Jiang Ying, Liao Xiangyu, Chen Qiongjun, Xiong Liang, Liu Yue, Liu Tong. Buqi Huoxue Compounds intervene with the expression of related factors and autophagy related proteins in a rat model of cerebral ischemia/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1152-1158. |
| [6] | Li Shuai, Liu Hua, Shang Yonghui, Liu Yicong, Zhao Qihang, Liu Wen. Stress distribution on the maxilla when wearing the Twin-block appliance for Class II malocclusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 881-887. |
| [7] | Han Haihui, Meng Xiaohu, Xu Bo, Ran Le, Shi Qi, Xiao Lianbo. Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 968-977. |
| [8] | Yu Shuangqi, Ding Fan, Wan Song, Chen Wei, Zhang Xuejun, Chen Dong, Li Qiang, Lin Zuoli. Effects of polylactic acid-glycolic acid copolymer/lysine-grafted graphene oxide nanoparticle composite scaffolds on osteogenic differentiation of MC3T3 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 707-712. |
| [9] | Zhang Yuhang, Zeng Yuning, Zeng Jindi, Lu Yixuan, Ye Hui, Ji Jianxin. Accuracy of modified implant template of assisted implantation in missing second molars [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 738-744. |
| [10] | Li Mingzhe, Ye Xiangling, Wang Bing, Yu Xiang. Preparation and osteogenic properties of liquid crystal display light-cured polylactic acid scaffold loaded with nano-tantalum [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 670-677. |
| [11] | Xiao Fang, Huang Lei, Wang Lin. Magnetic nanomaterials and magnetic field effects accelerate bone injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 827-838. |
| [12] | Peng Yong, Hu Jiangping, Zhu Huan. Effects of low-load blood flow restriction exercise and high-intensity resistance exercise on the thigh microcirculation function of athletic young men [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 393-401. |
| [13] | Li Zhenchao, Du Xiling, Han Zhixin, Niu Dawei, Fan Changwei. Mechanism by which exogenous basic fibroblast growth factor promotes wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2243-2251. |
| [14] | Zhang Xingzhou, Wei Ming, Dong Guoqiang, Du Wei, Luo Yiwen, Zhang Nan . Mechanism of postoperative abdominal adhesion formation and therapeutic prospect of mesenchymal stem cell exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 147-155. |
| [15] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||