Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (30): 4802-4808.doi: 10.12307/2023.547
Previous Articles Next Articles
Effects of hydrogels loaded with hepatocyte growth factor on myocardial infarction
Wang Nanfeng1, 2, Shen Shengmei2, Zhang Peisheng1, Teng Wei3
- 1The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou 450015, Henan Province, China; 2Nanshi Hospital of Nanyang, Nanyang 473000, Henan Province, China; 3The First Affiliated Hospital of Henan University, Kaifeng 410200, Henan Province, China
-
Received:2022-07-29Accepted:2022-09-09Online:2023-10-28Published:2023-04-01 -
Contact:Zhang Peisheng, Chief physician, The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou 450015, Henan Province, China -
About author:Wang Nanfeng, The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou 450015, Henan Province, China; Nanshi Hospital of Nanyang, Nanyang 473000, Henan Province, China -
Supported by:The Medical Science and Technology Foundation of Henan Province, No. LHGJ20190506 (to TW)
CLC Number:
Cite this article
Wang Nanfeng, Shen Shengmei, Zhang Peisheng, Teng Wei. Effects of hydrogels loaded with hepatocyte growth factor on myocardial infarction[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4802-4808.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

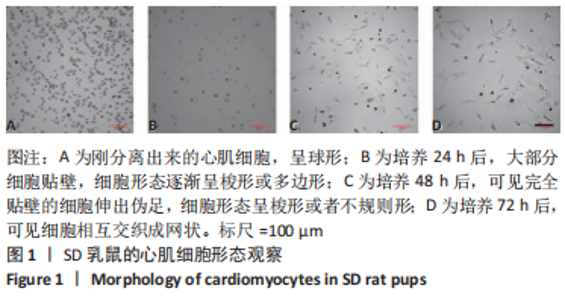
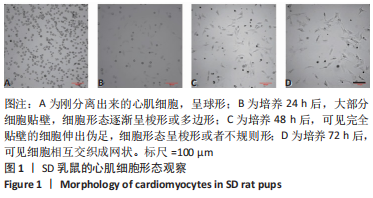
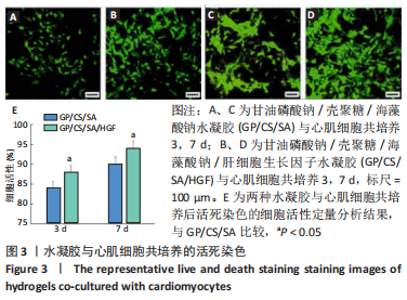
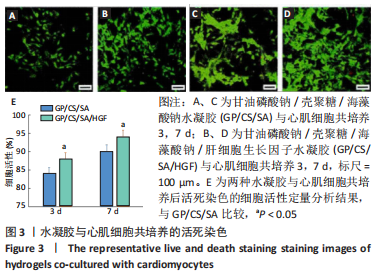
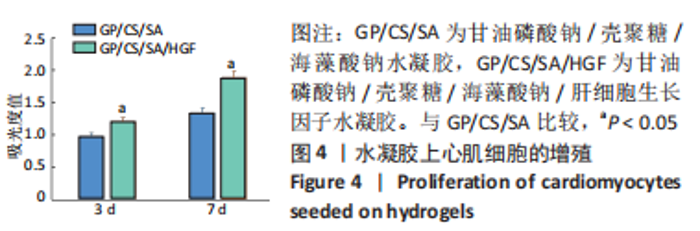
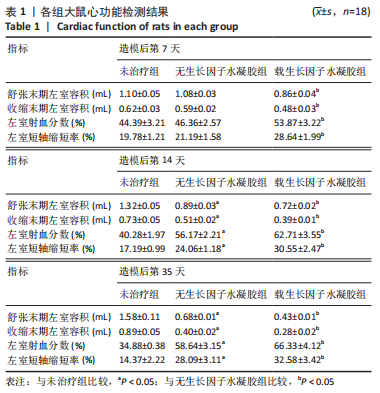
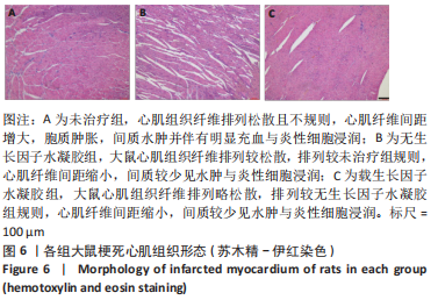
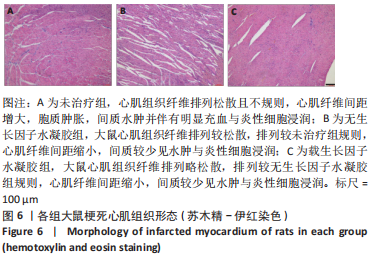
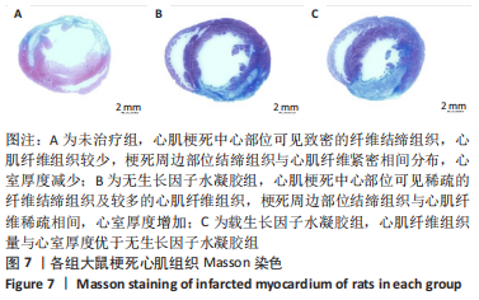
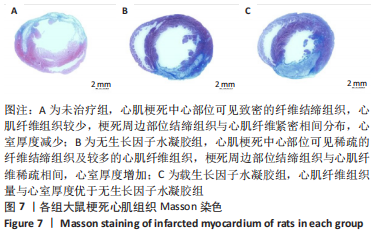
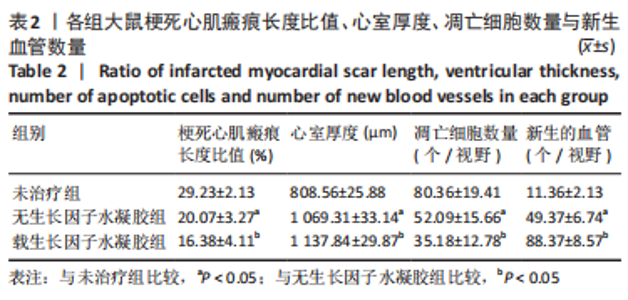
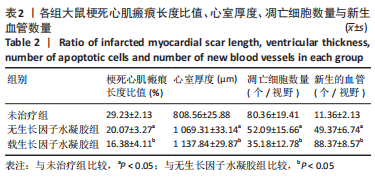
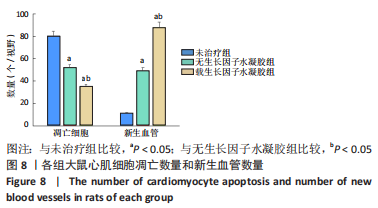
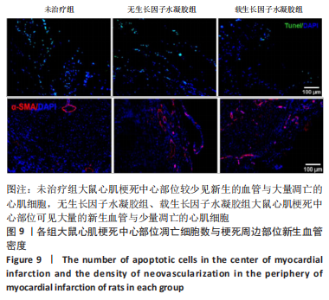
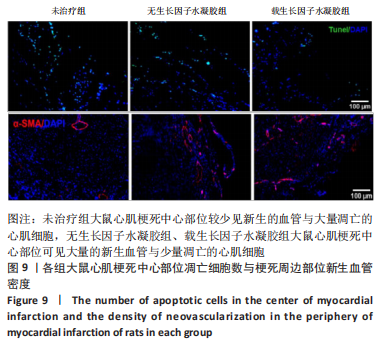
2.2 水凝胶材料的细胞相容性 扫描电镜下可见GP/CS/SA/HGF水凝胶具有均匀的多孔结构,孔洞之间相互连通,孔径100-300 μm,见图2。活死染色显示,两组水凝胶上的心肌细胞状态良好,大量发出绿色荧光的活细胞黏附在水凝胶表面,较少见发出红色荧光的死细胞,并且随着培养时间的延长,水凝胶表面的细胞数量增加,伸展良好,且连接成片,GP/CS/SA/HGF水凝胶上的细胞数量多于GP/CS/SA水凝胶上的细胞数量,见图3。CCK-8实验检测结果显示,随着培养时间的延长,两组水凝胶上的心肌细胞增殖吸光度值升高,同时GP/CS/SA/HGF水凝胶上的细胞吸光度值高于GP/CS/SA水凝胶,见图4。"

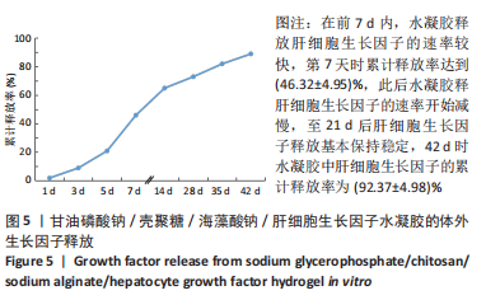
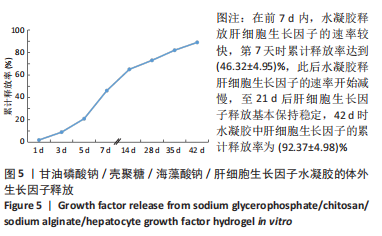
| [1] SAITO Y, TANAKA A, NODE K, et al. Uric acid and cardiovascular disease: A clinical review. J Cardiol. 2021;78(1):51-57. [2] SOPPERT J, LEHRKE M, MARX N, et al. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. 2020;159:4-33. [3] KAPUR NK, THAYER KL, ZWECK E. Cardiogenic Shock in the Setting of Acute Myocardial Infarction. Methodist Debakey Cardiovasc J. 2020;16(1):16-21. [4] MITSIS A, GRAGNANO F. Myocardial Infarction with and without ST-segment Elevation: a Contemporary Reappraisal of Similarities and Differences. Curr Cardiol Rev. 2021;17(4):e230421189013. [5] SUN J, SHEN H, SHAO L, et al. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther. 2020;11(1): 373. [6] YAO X, YANG W, REN Z, et al. Neuroprotective and Angiogenesis Effects of Levetiracetam Following Ischemic Stroke in Rats. Front Pharmacol. 2021;12:638209. [7] LIANG C, ZHANG T, SHI XL, et al. Modified Renshen Yangrong decoction enhances angiogenesis in ischemic stroke through promotion of MicroRNA-210 expression by regulating the HIF/VEGF/Notch signaling pathway. Brain Behav. 2021;11(8):e2295. [8] DIKICI S, BULLOCK AJ, YAR M, et al. 2-deoxy-d-ribose (2dDR) upregulates vascular endothelial growth factor (VEGF) and stimulates angiogenesis. Microvasc Res. 2020;131:104035. [9] QAYYUM AA, JOSHI FR, LUND LD, et al. Vascular endothelial growth factor therapy in ischaemic heart disease. Ugeskr Laeger. 2020;182(44): V05200344. [10] JEONG HM, WEON KY, SHIN BS, et al. 3D-Printed Gastroretentive Sustained Release Drug Delivery System by Applying Design of Experiment Approach. Molecules. 2020;25(10):2330. [11] KAMAL MM, AKTER S, AL HAGBANI T, et al. Sustained release of curcumin self-emulsifying drug delivery system (SEDDS) from solvent-cast Soluplus® films. Pharm Dev Technol. 2021;26(10):1102-1109. [12] QIU S, GAO J, LIU J, et al. Study on Novel Nanoparticle Slow-Release Drugs for Moyamoya Disease. J Nanosci Nanotechnol. 2021;21(2): 1008-1017. [13] MONTAGNAT OD, WEBSTER GR, BULITTA JB, et al. Lessons learned in the development of sustained release penicillin drug delivery systems for the prophylactic treatment of rheumatic heart disease (RHD). Drug Deliv Transl Res. 2018;8(3):729-739. [14] MADONNA R, ROKOSH G, DE CATERINA R, et al. Hepatocyte growth factor/Met gene transfer in cardiac stem cells--potential for cardiac repair. Basic Res Cardiol. 2010;105(4):443-452. [15] O’NEILL HS, O’SULLIVAN J, PORTEOUS N, et al. A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. J Tissue Eng Regen Med. 2018;12(1):e384-e394. [16] ZHANG Z, LONG C, GUAN Y, et al. Hepatocyte growth factor intervention to reduce myocardial injury and improve cardiac function on diabetic myocardial infarction rats. Eur J Histochem. 2020;64(s2):3142. [17] 陈慧敏,宋婷婷,吴齐越,等.海藻酸钠壳聚糖支架复合成骨细胞特异性多肽修复兔颅骨缺损[J].安徽医科大学学报,2017,52(4): 504-507 [18] LV K, LI Q, ZHANG L, et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics. 2019;9(24):7403-7416. [19] LI Y, CHEN X, JIN R, et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci Adv. 2021; 7(9):eabd6740. [20] CHEN R, ZHU C, XU L, et al. An injectable peptide hydrogel with excellent self-healing ability to continuously release salvianolic acid B for myocardial infarction. Biomaterials. 2021;274:120855. [21] RUVINOV E, COHEN S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv Drug Deliv Rev. 2016;96:54-76. [22] LLC BB. IK-5001 for the prevention of remodeling of the ventricle and congestive heart failure after acute myocardial infarction(PRESERVATION 1). Study NCT012265 63 Available at: http://www.ClinicalTrials.gov 2010 (Accessed July 16, 2015). [23] MCCARTHY PC, ZHANG Y, ABEBE F. Recent Applications of Dual-Stimuli Responsive Chitosan Hydrogel Nanocomposites as Drug Delivery Tools. Molecules. 2021;26(16):4735. [24] ERICKSON CB, NEWSOM JP, FLETCHER NA, et al. In vivo degradation rate of alginate-chitosan hydrogels influences tissue repair following physeal injury. J Biomed Mater Res B Appl Biomater. 2020;108(6): 2484-2494. [25] 王晓,郝新青,王小萌,等.壳聚糖/海藻酸钠水凝胶顺序释放BMP/WNT信号通路激活剂促成骨细胞分化的体外实验研究[J].口腔颌面外科杂志,2021,31(1):9-15 [26] GHOLAMI M, GILANPOUR H, SADEGHINEZHAD J, et al. Facile fabrication of an erythropoietin-alginate/chitosan hydrogel and evaluation of its local therapeutic effects on spinal cord injury in rats. Daru. 2021; 29(2):255-265. [27] 王秀力,姜世强,刘颖,等.慢病毒介导Wnt蛋白5a基因过表达对骨髓间充质干细胞移植治疗心肌梗死大鼠的影响[J].中华实验外科杂志,2021,38(9):1720-1723 [28] 刘化进,马江伟,乔增勇,等.血清VEGF、HGF在AMI阿托伐他汀强化治疗干预时的变化及对AMI预后的预测[J].贵州医药,2018, 42(11):1389-1391 [29] KAMBE Y, YAMAOKA T. Biodegradation of injectable silk fibroin hydrogel prevents negative left ventricular remodeling after myocardial infarction. Biomater Sci. 2019;7(10):4153-4165. [30] GUO W, FENG W, HUANG J, et al. Supramolecular Self-Assembled Nanofibers Efficiently Activate the Precursor of Hepatocyte Growth Factor for Angiogenesis in Myocardial Infarction Therapy. ACS Appl Mater Interfaces. 2021;13(19):22131-22141. [31] SALA V, GALLO S, GATTI S, et al. Cardiac concentric hypertrophy promoted by activated Met receptor is mitigated in vivo by inhibition of Erk1,2 signalling with Pimasertib. J Mol Cell Cardiol. 2016;93:84-97. [32] WANG Z, FEI S, SUO C, et al. Antifibrotic Effects of Hepatocyte Growth Factor on Endothelial-to-Mesenchymal Transition via Transforming Growth Factor-Beta1 (TGF-β1)/Smad and Akt/mTOR/P70S6K Signaling Pathways. Ann Transplant. 2018;23:1-10. |
| [1] | Liu Xiaolin, Mu Xinyue, Ma Ziyu, Liu Shutai, Wang Wenlong, Han Xiaoqian, Dong Zhiheng. Effect of hydrogel-loaded simvastatin microspheres on osteoblast proliferation and differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 998-1003. |
| [2] | Li Cheng, Zheng Guoshuang, Kuai Xiandong, Yu Weiting. Alginate scaffold in articular cartilage repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1080-1088. |
| [3] | Zhou Heshan, Tan Longwang, Liu Chuang, Zhang Chi. Pretreatment methods improve the role of exosomes in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5394-5403. |
| [4] | Huang Miao, Wu Xiesheng, Huang Wenshi, Huang Haina, Chen Longyun, Peng Weijie. Properties, functions and applications of three-dimensional models of bone for drug screening [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(31): 5071-5077. |
| [5] | Peng Fengli, Li Chaofu, Shi Bei. Application of the nanovesicle delivery system in cardiovascular diseases [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4862-4868. |
| [6] | Zhang Qingmei, Zhang Lupeng, Du Xiujuan, Li Bing. Application of carbon dots-based materials in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4883-4889. |
| [7] | Jiang Honghui, Kong Yuanyuan, Liu Jing, Wang Zhihong. Preparation and applications of decellularized extracellular matrix bioink in cardiovascular fields [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4904-4911. |
| [8] | Li Yue, Lyu Yan, Feng Wanying, Song Yang, Yan Yu, Guan Yongge. Preparation of hyperoside nanoparticles to repair endometrial injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 360-366. |
| [9] | Wei Qin, Amanguli·Ruze, Chen Bingxin, Zhao Ling, Zhao Banghao, Jiang Tao, Zhang Chun, Li Zhiqiang, Gao Xiaoming, Duan Mingjun. Relaxin protects myocardial microvascular endothelial cells from hypoxia-reoxygenation injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(28): 4519-4524. |
| [10] | He Wenfeng, Xue Cheng, Zheng Jiankang, Shuai Zhuang, Yue Rongchuan. Effects of icariin combined with injectable chitosan/collagen composite hydrogel on angiogenesis in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3992-3998. |
| [11] | Li Shaoping, Yang Yuqing, Xiao Wenyundeng, Yin Lulu, Liu Libo, Liu Ying, Sun Yifan, Chen Zhiyu. Preparation and characterization of nano-hydroxyapatite/aspirin/polyvinyl alcohol/gelatin/sodium alginate hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3956-3963. |
| [12] | Cao Congcong, Ling Gengfei, Yang Chunhua. Local injection of ginsenoside Rg1 nanoparticles in the treatment of myocardial infarction in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3977-3983. |
| [13] | Yi Jiafeng, Liu Yubo, Li Chao, Wang Xing, Chai Wei. Lubrication mechanism in articular cartilage and tribological application of biomimetic cartilage materials [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 4075-4084. |
| [14] | Lu Pengfei, Shi Weili. NICD/Hes1 affects angiogenesis in the ischemic myocardium of myocardial infarction mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(23): 3635-3639. |
| [15] | Yu Zhijun, Zhang Guijuan, Chen Lixin, Yang Senlin, Cui Yuxiang. Effects of Caveolin-1 on cardiac microvasculature, ventricular remodeling and mitochondrial respiratory function in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(23): 3660-3666. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||