Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (10): 1618-1625.doi: 10.12307/2023.267
Previous Articles Next Articles
Opportunities and challenges of COVID-19 therapy using mesenchymal stem cells and their exosomes
Zhang Yuqing, Wu Jun
- Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Institute of Biochemistry and Cell Biology/Cell Bank, Type Culture Collection of Chinese Academy of Sciences, Shanghai 200032, China
-
Received:2022-03-24Accepted:2022-05-13Online:2023-04-08Published:2022-09-09 -
Contact:Zhang Yuqing, Master, Junior engineer, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Institute of Biochemistry and Cell Biology/Cell Bank, Type Culture Collection of Chinese Academy of Sciences, Shanghai 200032, China -
About author:Zhang Yuqing, Master, Junior engineer, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Institute of Biochemistry and Cell Biology/Cell Bank, Type Culture Collection of Chinese Academy of Sciences, Shanghai 200032, China -
Supported by:grants from National Science and Technology Resource Sharing Service Platform, No. E019380101; Technical Support Talent of Chinese Academy of Sciences, No. E219380101; Strategic Biological Resource Program of Chinese Academy of Sciences (KFJ-BRP) Biological Genetic Resource Bank, No. O819S31942 (to ZYQ)
CLC Number:
Cite this article
Zhang Yuqing, Wu Jun. Opportunities and challenges of COVID-19 therapy using mesenchymal stem cells and their exosomes[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1618-1625.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
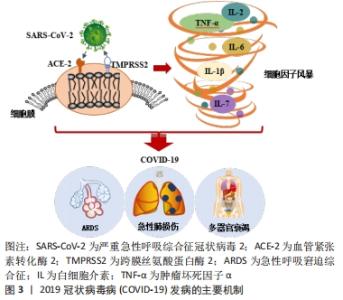
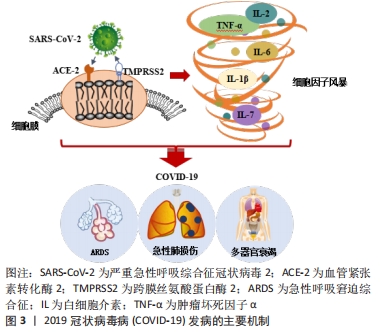
2.1 COVID-19的发病机制 血管紧张素转化酶2(angiotensin-converting enzyme 2,ACE2)与跨膜丝氨酸蛋白酶2(transmembrane protease serine 2,TMPRSS2)在SARS-COV-2病毒入侵中发挥关键作用。SARS-COV-2病毒的表面有4种与其他冠状病毒相似的结构蛋白,分别是刺突蛋白(Spike protein,S蛋白)、包膜蛋白(Envelope protein,E蛋白)、膜蛋白(Membrane protein,M蛋白)、核衣壳蛋白(Nucleocapsid,N蛋白)等。S蛋白具备2个受体结合亚基S1与S2,S1可通过其本身的受体结构域来识别宿主细胞表面的特定受体,即ACE2受体[17]。当SARS-COV-2病毒侵入人体时,ACE2受体就像一个“门把手”,SARS-COV-2病毒抓住它并打开通往受体细胞的大门。实验结果表明,SARS-COV-2病毒表面的S蛋白以相同的亲和力结合ACE2受体,比SARS病毒的亲和力高20倍[18],这可能也是COVID-19为什么更易于传播的一大原因。此外,最新的研究结果显示:奥密克戎毒株因其表面的S蛋白氨基酸位点发生突变,导致其与靶细胞表面的ACE2受体结合的亲和力与活跃度比原始的SARS-COV-2病毒高出10倍水平,其隐匿性和传染性相较于原始的SARS-COV-2毒株也存在显著增加,这也是今年COVID-19全球感染人数突然剧增的主要原因[19]。同时,以往的研究也证实在SARS-COV-2病毒与宿主细胞融合内化时,TMPRSS2重组蛋白也会参与S蛋白的切割过程,诱导病毒与靶细胞更快的融合[20]。紧接着,SARS-COV-2病毒基因组进入宿主细胞,开始复制、成熟并离开宿主细胞以感染新的健康细胞,此时人体被确诊感染了COVID-19。由于ACE2受体和TMPRSS2重组蛋白广泛存在于肾脏、血管、心脏中,尤其是在Ⅱ型肺泡细胞和内皮细胞中含量尤为丰富[21],所以肺血管更容易受到SARS-COV-2病毒诱导的炎症损伤[22]。SARS-COV-2病毒从上呼吸道进入呼吸系统,最终感染肺泡细胞,引起肺泡壁毛细血管通透性增加、肺表面活性物质减少,同时淋巴细胞和单核细胞也开始浸润,继而诱导产生ARDS及急性肺损伤等临床症状[7]。此外,除呼吸道外,SARS-COV-2病毒感染还会导致身体其他部位的受损,如心血管系统等。因此,除了呼吸功能障碍外,COVID-19患者还可能会出现严重的心肌炎或心内膜炎等症状,这些都严重威胁着人类的生命健康[23]。 另外,COVID-19患者病情加重的主要原因还有过量炎症因子的产生。SARS-COV-2病毒进入宿主细胞取决于与其受体ACE2的结合,因此肾素-血管紧张素系统和各种炎症级联反应与COVID-19的病理生理学有关[24]。在感染宿主细胞的同时,SARS-CoV-2病毒还会激活COVID-19患者体内先天性和适应性免疫反应[25]。随后免疫效应细胞开始释放出大量的炎症因子和趋化因子,如肿瘤坏死因子α、白细胞介素1β、白细胞介素6、白细胞介素7和单核细胞趋化蛋白1(monocyte chemotactic protein-1,MCP-1)等[26],进而引起人体内过度的免疫反应。适当的炎症反应会抑制SARS-COV-2病毒的扩散并消除病毒感染,而过度和不受控制的免疫反应则会造成所谓的细胞因子风暴也叫细胞因子释放综合征,进而引起COVID-19患者出现ARDS症状,同时伴随着各种临床表现,如高烧、肝脾肿大、血细胞减少、中枢神经系统异常、低白蛋白血症和毛细血管渗漏综合征等症状[27-28]。细胞因子的过度表达也会引起COVID-19患者出现肺水肿并损害肺部气体交换和氧代谢[16],直接加速了肺损伤的程度,同时还可能会引发潜在的心脏损伤、多器官继发感染和死亡等现象。图3为COVID-19发病的主要机制图。目前,世界各地正在探索使用适当的免疫抑制剂和免疫调节剂来解决COVID-19的潜在炎症并发症,这可能会成为改善COVID-19重症患者临床症状并降低COVID-19死亡率的最有效策略之一。 "
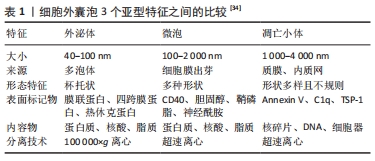
2.2 间充质干细胞及其外泌体作为COVID-19的潜在疗法 目前SARS-COV-2病毒主流变异株——奥密克戎毒株的出现,使得COVID-19患者多呈现为无症状或轻型症状,但是依旧无法避免一些病情严重的患者可能会出现ARDS及肺损伤等症状,且目前没有特异的治疗策略对其进行有效的治疗以及损伤后的组织功能恢复[6]。间充质干细胞因其具有从中胚层向不同谱系转化的能力而被划分为多能干细胞[29]。间充质干细胞具有多种独特的特性,包括组织再生能力、抗炎作用、免疫逃避特性以及与各种细胞内/外途径的相互作用,使间充质干细胞成为治疗不同病理状况的新型方法之一,在细胞治疗、再生医学和组织修复等方面均具有重要的意义[30]。同时,它们更容易获得,可以从脐带、脐血、经血、骨髓、胎盘、脂肪等多种组织中分离和纯化[31],间充质干细胞的有效性和安全性也已在多项临床试验研究中得到显著验证[32]。尤其在免疫调节方面,间充质干细胞可充当先天性和适应性免疫系统的免疫调节剂,通过影响炎性巨噬细胞并促进其向修复表型的极化来显示出其治疗效 果[33]。在之前发表的几项研究中,发现间充质干细胞还可以抑制肺浸润和肺水肿的溶解,并且具有抑制免疫反应和体外分化为Ⅱ型肺泡上皮细胞的能力[34]。在对ARDS患者进行检查的Ⅱ期临床试验中,间充质干细胞也被证明具有抗炎活性,能够减轻COVID-19相关的ARDS程度,被证明是慢性呼吸功能障碍和肺纤维化的潜在前瞻性疗法之一[13]。 此外,间充质干细胞还可以通过旁分泌作用来发挥其治疗效果[35]。间充质干细胞的旁分泌作用主要由其衍生的细胞外囊泡来实现。这些囊泡参与细胞间传递、细胞信号转导以及在体内不同距离处改变细胞或组织代谢。根据其大小、表面标志物和细胞生成机制等方面的不同,细胞外囊泡主要分为3个亚型:外泌体、微泡和凋亡小体[34],3者的特征比较详见表1。 "
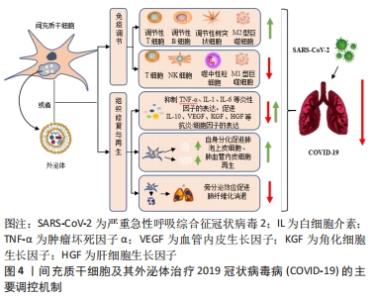
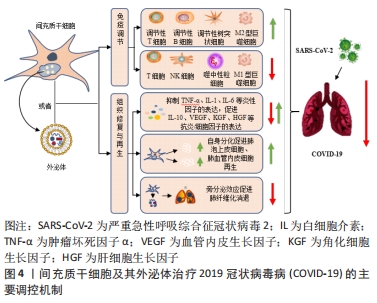
外泌体作为间充质干细胞衍生的主要囊泡之一,其具有保护各种组织器官损伤、再生和修复的能力,这可能在ARDS的治疗中同样发挥着重要作用[16]。此外研究显示:相较于细胞治疗组,利用间充质干细胞衍生的外泌体作为无细胞疗法具有优于细胞疗法的显著优势,包括确定剂量和效力、避免侵入性细胞活检、低免疫原性、高稳定性、更好穿过血脑屏障的能力以及更易于获取与储存等方面[36]。同时,这些外泌体具有双脂质膜结构和更高的生物相容性,也赋予了这些细胞外囊泡可作为药物递送系统的潜力,其在COVID-19大流行病中发挥药理学和治疗中介方面,也获得了足够多的支持[37-38]。它们不仅可以减轻患者体内过度的炎症免疫反应,还可以修复肺损伤。因此,间充质干细胞及其衍生的外泌体可以单独使用,也可以与其他免疫治疗药物联合使用,最终使COVID-19患者从中受益。 2.3 间充质干细胞及其外泌体治疗COVID-19的主要调控机制 如前所述,炎症因子的过度产生从而引起体内不受控制的免疫反应,继而出现的ARDS症状及急性肺损伤是导致COVID-19患者病情加重甚至出现死亡的主要原因[16]。而间充质干细胞及其外泌体所具有的强大的免疫调节及组织修复与再生能力,使其在治疗COVID-19患者方面显示出极大的优势。 2.3.1 免疫调节 目前研究证实:间充质干细胞在严重的炎症条件下,具有一定的免疫调节能力。针对COVID-19治疗方面,间充质干细胞可通过抑制自然杀伤细胞的活化、树突状细胞的成熟以及T细胞与B细胞的过度增殖等过程,同时促进调节性T细胞亚群的生成以及M1型促炎巨噬细胞向M2型抗炎巨噬细胞表型的转变,从而降低COVID-19患者体内过度激活的免疫应答水平[39-40]。此外,间充质干细胞及其外泌体还携带着复杂的生物物质,包括核酸、miRNA、脂质和蛋白质等生物活性分子,这些分子被传送到相邻和远处的细胞,从而改变受体细胞的命运[41]。最近发表的几项研究表明,间充质干细胞通过其衍生外泌体所释放的多种miRNA分子,如miR-21、miR-24、miR-124、miR-let-7b、miR-let-7c、miR125b等,可通过作用于Toll样受体4、转化生长因子β2、Smad2、转化生长因子β受体1等炎症相关蛋白,从而减少白细胞介素7、白细胞介素2、白细胞介素6等炎症细胞因子的分泌,降低患者体内的炎症水平[41-42]。间充质干细胞还通过增加患者体内白细胞介素10和血管内皮生长因子的分泌,降低肿瘤坏死因子α的表达水平,减少患者肺内噬中性粒细胞的流入和肺充血程度[9,43],最终逆转由SARS-CoV-2引起的细胞因子风暴,从而有效治疗COVID-19患者体内ARDS和急性肺损伤等症状。此外,BARI等[44]的一项研究表明,间充质干细胞衍生的外泌体在其表面表达α1-抗胰蛋白酶,这种结构能够抑制噬中性粒细胞衍生的蛋白水解酶,同时还具有抗炎和免疫调节作用,有利于保护肺上皮细胞。研究表明间充质干细胞衍生的外泌体也能够促进巨噬细胞表型从M1型向M2型的转变[45],通过增加白细胞介素10的分泌来调节肺部树突状细胞的免疫抑制功能,进而阻止肺部M1型巨噬细胞和树突状细胞相关的免疫反应[46-48],改善SARS-CoV-2感染后的不良后果。由此,这也预示着外泌体在加速受损肺组织的愈合方面具有着巨大的应用潜力,同时也为COVID-19患者的治疗提出了更有希望的见解。 2.3.2 组织修复与再生 临床结果发现:部分COVID-19重症患者的肺部除了出现大面积炎症、肺泡出现损伤之外,还会出现不同程度的肺纤维化症状。这可能是由于大量炎症因子的刺激导致肺部成纤维细胞出现异常增殖,之后产生大量的胶原和细胞外基质沉积导致,如果治疗不及时也会对COVID-19患者的肺部造成永久性的损伤[49-50]。以往研究表明,间充质干细胞是治疗肺纤维化的前瞻性疗法之一,通过迁移至损伤部位,修复组织的同时通过分化产生肺泡上皮细胞与肺血管内皮细胞从而发挥其组织再生作用[50]。除此之外,间充质干细胞及其外泌体通过分泌白细胞介素1受体拮抗剂、基质金属蛋白酶9、粒细胞-巨噬细胞集落刺激因子等物质,抑制肺血管内皮细胞凋亡的同时,能够特异刺激肺上皮细胞的新陈代谢等生理过程,包括肺泡上皮细胞的再生、分化和迁移等,从而促进肺损伤部位的功能恢复与再生[16]。同时,外泌体中富含的miR-145和相关蛋白质的存在也显著促进了损伤肺组织的功能维持和再生[16],这也为间充质干细胞及其外泌体促进组织修复与再生提供了新的见解。 除此之外,最新的临床研究结果表明:肺血管内皮细胞在SARS-CoV-2感染过程中也可作为治疗靶点发挥重要作用[23]。间充质干细胞及其外泌体通过分泌人类生长因子和血管内皮生长因子保护ARDS患者肺血管内皮细胞与肺泡上皮细胞的同时,可增加呼吸道毛细血管的通透性,提高血管内皮钙黏蛋白的表达水平,减少内皮细胞中促炎因子的分泌,从而增强患者体内肺泡上皮-内皮屏障的稳定,进而发挥其组织修复作用,可用作COVID-19患者有希望的治疗选择之一[23,51-53]。图4为间充质干细胞及其外泌体治疗COVID-19的主要调控机制。 "

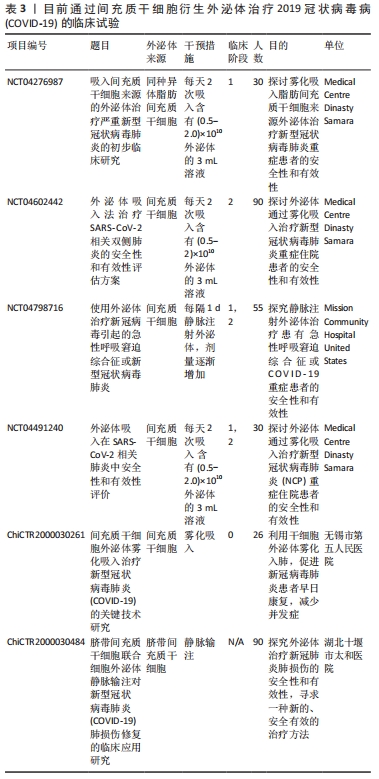
2.4 间充质干细胞及其外泌体治疗COVID-19的最新进展 目前很多医疗机构针对COVID-19特效药物的研发一直在进行当中,但是依旧没有针对COVID-19重症患者的特异治疗策略。常规治疗策略主要包括感染预防、辅助性护理等(包括补充氧气和机械通气支持)[48,54]。全球都在评估针对COVID-19的药物,包括抗病毒药物、抗疟疾药物、单克隆抗体等,尽管这些治疗策略可以促进患者康复和提高存活率,但它们并不能完全恢复由SARS-CoV-2引起的肺损伤症状。此外,一系列抗炎药已被测试可抑制重症患者因免疫反应恶化而引起的细胞因子风暴和多器官衰竭,但效果也并不显著。研究人员正在开发针对SARS-CoV-2感染的潜在疗法,包括基于间充质干细胞及其衍生外泌体的COVID-19治疗等[55-58]。以往的文献报道已经证实,间充质干细胞治疗在ARDS患者中具有安全性和潜在疗效[13],其衍生的外泌体对ARDS症状也具有积极的治疗作用,可通过将mRNA和miRNA转移到肺组织来抑制ARDS患者体内的细胞因子风暴程度[53,59]。在非感染性急性肺损伤和肺部细菌性败血症的临床前模型中,间充质干细胞衍生的外泌体也显示出由miRNA和抑制性mRNA介导的保护作用[60]。由于SARS-CoV-2的发病机制与之前遇到的大多数引起ARDS和急性肺损伤的病毒相似,所以关于间充质干细胞及其衍生外泌体的临床前和临床修复功能的研究对其治疗COVID-19有很好的提示性,两者治疗的有效性也应该在SARS-CoV-2的背景下进行探索。所以科学家们开始假设:通过使用间充质干细胞或其衍生的外泌体,可以在COVID-19诱导的肺炎中预防肺纤维化。由于间充质干细胞及其衍生的外泌体具有潜在的抗炎作用,也可以通过输液的形式用于治疗COVID-19确诊病例,以预防患者体内过度炎症反应的发生。表2是目前国际临床试验资料库(ClinicalTrials.gov)中显示通过间充质干细胞治疗COVID-19且状态显示“完成”的临床试验。"


在临床研究中,北京佑安医院采取生物治疗的方法,将骨髓间充质干细胞植入7例COVID-19患者体内,临床结果显示所有患者在骨髓间充质干细胞移植后第2天开始,其免疫功能和临床指标均得到明显改善,症状较轻的2例患者在第10天已达到出院指标[61]。同期也有研究者通过静脉注射的方式将脐带间充质干细胞植入1例65岁的COVID-19重症患者体内使其最终得到有效救治[62]。此外,SENGUPTA等[59]也进行了第一阶段临床试验,在24例患有中-重度ARDS的COVID-19感染受试者中测试了同种异体骨髓间充质干细胞衍生外泌体的治疗效果,结果显示外泌体给药后,在提升COVID-19患者肺部血氧饱和度和氧合指数的同时,还下调了患者体内的细胞因子水平,最终使得中重度COVID-19患者的存活率达到83% (其中4例因无关治疗而死亡,71%的患者从COVID-19中完全康复),肺部损伤也得到进一步恢复。由此可见,源自间充质干细胞谱系的外泌体也可以为COVID-19感染提供有希望的治疗方法[3]。目前一些通过使用间充质干细胞衍生的外泌体治疗COVID-19的临床试验也正在进行当中,汇总见表3。详细信息可通过国际临床试验资料库(ClinicalTrials.gov)与中国临床试验注册中心(http://www.chictr.org.cn)进行搜索。 "
| [1] YESUDHAS D, SRIVASTAVA A, GROMIHA M M. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection. 2021;49(2):199-213. [2] DA COSTA VG, MORELI ML, SAIVISH MV. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch Virol. 2020;165(7): 1517-1526. [3] NABEL KG, CLARK SA, SHANKAR S, et al. Structural basis for continued antibody evasion by the SARS-CoV-2 receptor binding domain. Science. 2022;375(6578):eabl6251. [4] VITIELLO A, FERRARA F, AUTI AM, et al. Advances in the Omicron variant development. J Intern Med. 2022 Mar 15. doi: 10.1111/joim.13478. Online ahead of print. [5] ARAF Y, AKTER F, TANG YD, et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94(5):1825-1832. [6] NGAI HW, KIM DH, HAMMAD M, et al. Stem Cell-based therapies for COVID-19-related acute respiratory distress syndrome. J Cell Mol Med. 2022;26(9):2483-2504. [7] YAN YY, ZHOU WM, WANG YQ, et al. The Potential Role of Extracellular Vesicles in COVID-19 Treatment: Opportunity and Challenge. Front Mol Biosci. 2021;8:699929. [8] XU X, HAN M, LI T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970-10975. [9] MEHTA P, MCAULEY DF, BROWN M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. [10] MAZINI L, ROCHETTE L, AMINE M, et al. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int J Mol Sci. 2019;20(10):2523. [11] MAZINI L, ROCHETTE L, ADMOU B, et al. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020;21(4):1306. [12] LAFFEY JG, MATTHAY MA. Fifty Years of Research in ARDS. Cell-based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am J Respir Crit Care Med. 2017;196(3):266-273. [13] MATTHAY MA, CALFEE CS, ZHUO H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154-162. [14] DAULETOVA M, HAFSAN H, MAHHENGAM N, et al. Mesenchymal stem cell alongside exosomes as a novel cell-based therapy for COVID-19: A review study. Clin Immunol. 2021;226:108712. [15] O’LEARY VB, DOLLY OJ, HÖSCHL C, et al. Unpacking Pandora From Its Box: Deciphering the Molecular Basis of the SARS-CoV-2 Coronavirus. Int J Mol Sci. 2020;22(1):386. [16] WRAPP D, WANG N, CORBETT KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260-1263. [17] SCHELLER N, HEROLD S, KELLNER R, et al. Proviral MicroRNAs Detected in Extracellular Vesicles From Bronchoalveolar Lavage Fluid of Patients With Influenza Virus-Induced Acute Respiratory Distress Syndrome. J Infect Dis. 2019;219(4):540-543. [18] HAMMING I, TIMENS W, BULTHUIS ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-637. [19] YIN W, XU Y, XU P, et al. Structures of the Omicron spike trimer with ACE2 and an anti-Omicron antibody. Science. 2022;375(6584):1048-1053. [20] ZHAO Y, ZHAO Z, WANG Y, et al. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202(5):756-759. [21] GUZIK TJ, MOHIDDIN SA, DIMARCO A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666-1687. [22] BOURGONJE AR, ABDULLE AE, TIMENS W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228-248. [23] YANG L, LIU S, LIU J, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):128. [24] PEDERSEN SF, HO YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020; 130(5):2202-2205. [25] ENGLAND JT, ABDULLA A, BIGGS CM, et al. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2021;45:100707. [26] GAO YM, XU G, WANG B, et al. Cytokine storm syndrome in coronavirus disease 2019: A narrative review. J Intern Med. 2021;289(2):147-161. [27] CAPLAN AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650. [28] BABAJANI A, SOLTANI P, JAMSHIDI E, et al. Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer. Front Bioeng Biotechnol. 2020;8:748. [29] YOUSEFI DEHBIDI M, GOODARZI N, AZHDARI MH, et al. Mesenchymal stem cells and their derived exosomes to combat Covid-19. Rev Med Virol. 2022;32(2):e2281. [30] GOLCHIN A, FARAHANY TZ, KHOJASTEH A, et al. The Clinical Trials of Mesenchymal Stem Cell Therapy in Skin Diseases: An Update and Concise Review. Curr Stem Cell Res Ther. 2019;14(1):22-33. [31] WHELAN DS, CAPLICE NM, CLOVER AJP. Mesenchymal stromal cell derived CCL2 is required for accelerated wound healing. Sci Rep. 2020;10(1):2642. [32] GAZDIC M, VOLAREVIC V, ARSENIJEVIC N, et al. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep. 2015; 11(2):280-287. [33] RAHMATI S, SHOJAEI F, SHOJAEIAN A, et al. An overview of current knowledge in biological functions and potential theragnostic applications of exosomes. Chem Phys Lipids. 2020;226:104836. [34] DOYLE LM, WANG MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8(7):727. [35] YIN K, WANG S, ZHAO RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. [36] PASHOUTAN SARVAR D, SHAMSASENJAN K, AKBARZADEHLALEH P. Mesenchymal Stem Cell-Derived Exosomes: New Opportunity in Cell-Free Therapy. Adv Pharm Bull. 2016;6(3):293-299. [37] GAO F, CHIU SM, MOTAN DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7(1):e2062. [38] EL ANDALOUSSI S, MÄGER I, BREAKEFIELD XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013; 12(5):347-357. [39] JAMSHIDI E, BABAJANI A, SOLTANI P, et al. Proposed Mechanisms of Targeting COVID-19 by Delivering Mesenchymal Stem Cells and Their Exosomes to Damaged Organs. Stem Cell Rev Rep. 2021;17(1):176-192. [40] HE X, DONG Z, CAO Y, et al. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019; 2019:7132708. [41] KHEIRKHAH AH, SHAHCHERAGHI SH, LOTFI M, et al. Mesenchymal Stem Cell Derived-Exosomes as Effective Factors in Reducing Cytokine Storm Symptoms of COVID-19. Protein Pept Lett. 2021;28(8):945-952. [42] LIU S, PENG D, QIU H, et al. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020;11(1):169. [43] TEUWEN LA, GELDHOF V, PASUT A, et al. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389-391. [44] BARI E, FERRAROTTI I, DI SILVESTRE D, et al. Adipose Mesenchymal Extracellular Vesicles as Alpha-1-Antitrypsin Physiological Delivery Systems for Lung Regeneration. Cells. 2019;8(9):965. [45] YANG Y, CHEN QH, LIU AR, et al. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther. 2015;6:250. [46] LI X, GENG M, PENG Y, et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102-108. [47] DEVANEY J, HORIE S, MASTERSON C, et al. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 2015;70(7):625-635. [48] LEE JW, FANG X, GUPTA N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106(38): 16357-16362. [49] GARCIA-REVILLA J, DEIERBORG T, VENERO JL, et al. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front Immunol. 2020;11:2069. [50] CAI Q, YIN F, HAO L, et al. Research Progress of Mesenchymal Stem Cell Therapy for Severe COVID-19. Stem Cells Dev. 2021;30(9):459-472. [51] HAO Q, GUDAPATI V, MONSEL A, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Decrease Lung Injury in Mice. J Immunol. 2019; 203(7):1961-1972. [52] KIM J, YANG YL, JEONG Y, et al. Middle East Respiratory Syndrome-Coronavirus Infection into Established hDPP4-Transgenic Mice Accelerates Lung Damage Via Activation of the Pro-Inflammatory Response and Pulmonary Fibrosis. J Microbiol Biotechnol. 2020;30(3):427-438. [53] ZENG SL, WANG LH, LI P, et al. Mesenchymal stem cells abrogate experimental asthma by altering dendritic cell function. Mol Med Rep. 2015;12(2):2511-2520. [54] BAO L, DENG W, HUANG B, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830-833. [55] POPOWSKI KD, DINH PC, GEORGE A, et al. Exosome therapeutics for COVID-19 and respiratory viruses. View (Beijing). 2021:20200186. [56] MONSEL A, ZHU YG, GENNAI S, et al. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med. 2015;192(3):324-336. [57] LENG Z, ZHU R, HOU W, et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11(2):216-228. [58] LIANG B, CHEN J, LI T, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine (Baltimore). 2020;99(31):e21429. [59] SENGUPTA V, SENGUPTA S, LAZO A, et al. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29(12):747-754. [60] GUPTA A, KASHTE S, GUPTA M, et al. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Hum Cell. 2020;33(4):907-918. [61] GOLCHIN A, SEYEDJAFARI E, ARDESHIRYLAJIMI A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep. 2020;16(3): 427-433. [62] SHU L, NIU C, LI R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. [63] RAGHAV A, KHAN ZA, UPADHAYAY VK, et al. Mesenchymal Stem Cell-Derived Exosomes Exhibit Promising Potential for Treating SARS-CoV-2-Infected Patients. Cells. 2021;10(3):587. [64] WEN S, DOONER M, PAPA E, et al. Biodistribution of Mesenchymal Stem Cell-Derived Extracellular Vesicles in a Radiation Injury Bone Marrow Murine Model. Int J Mol Sci. 2019;20(21):5468. |
| [1] | Nong Fuxiang, Jiang Zhixiong, Li Yinghao, Xu Wencong, Shi Zhilan, Luo Hui, Zhang Qinglang, Zhong Shuang, Tang Meiwen. Bone cement augmented proximal femoral nail antirotation for type A3.3 intertrochanteric femoral fracturalysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-10. |
| [2] | Pan Zhongjie, Qin Zhihong, Zheng Tiejun, Ding Xiaofei, Liao Shijie. Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1441-1447. |
| [3] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [4] | Lian Shilin, Zhang Yan, Jiang Qiang, Zhang Hanshuo, Li Tusheng, Ding Yu. Interventional effects of whole blood and platelet-rich plasma with different preparation methods on nucleus pulposus cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1199-1204. |
| [5] | Xue Ting, Zhang Xinri, Kong Xiaomei. Mesenchymal stem cell therapy for pneumoconiosis using nanomaterials combined with multi-modal molecular imaging [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1133-1140. |
| [6] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [7] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [8] | Cui Lianxu, Jiang Wenkang, Lu Dahong, Xu Junrong, Liu Xiaocui, Wang Bingyun. Clinical-grade human umbilical cord mesenchymal stem cells affect the improvement of neurological function in rats with traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 835-839. |
| [9] | Li Qicheng, Deng Jin, Fu Xiaoyang, Han Na. Effects of bone marrow mesenchymal stem cells-derived exosomes on hypoxia-treated myoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 853-859. |
| [10] | Wang Min, Yin Xiushan, Wang Yingxi, Zhang Yan, Zhao Long, Xia Shuyue. Inhalation of bone marrow mesenchymal stem cells-derived exosomes alleviates inflammatory injury in chronic obstructive pulmonary disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 827-834. |
| [11] | Zhang Houjun, Deng Bowen, Jiang Shengyuan, Zhao Yi, Ren Jingpei, Xu Lin, Mu Xiaohong. Proteomic analysis of cerebrospinal fluid exosomes derived from cerebral palsy children [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 903-908. |
| [12] | Gao Ting, Ma Xiaohong, Li Xiaorong. Extraction and identification of exosomes from three different sources of ovarian granulosa cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 860-865. |
| [13] | Huang Guijiang, Ji Yuwei, Zhao Xin, Yang Yi, Zhao Yulan, Wang Peijin, Tang Wei, Jiao Jianlin. Effect and mechanism of different administration routes of placenta-derived mesenchymal stem cells in the treatment of tree shrews with osteoporotic fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 909-914. |
| [14] | Zhang Qijian, Xu Ximing. Acquisition and application of ectodermal mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 928-934. |
| [15] | Yuan Bo, Xie Lide, Fu Xiumei. Schwann cell-derived exosomes promote the repair and regeneration of injured peripheral nerves [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 935-940. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

