Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (3): 441-446.doi: 10.12307/2023.047
Previous Articles Next Articles
Research progress in platelet-rich fibrin in stomatology
Chen Jingqiao, Li Ying, Meng Maohua, Xu Xingxing, Wang Qinying, Wang Huan, Lu Jing, Shu Jiayu, Dong Qiang
- Affiliated Stomatological Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2021-09-01Accepted:2021-10-20Online:2023-01-28Published:2022-06-01 -
Contact:Dong Qiang, MD, Chief physician, Professor, Master’s supervisor, Affiliated Stomatological Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Chen Jingqiao, Master candidate, Affiliated Stomatological Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:the National Natural Science Foundation of China, No. 81860192 (to DQ); Guiyang Municipal Science and Technology Plan Project, No. [2017]3040 (to DQ)
CLC Number:
Cite this article
Chen Jingqiao, Li Ying, Meng Maohua, Xu Xingxing, Wang Qinying, Wang Huan, Lu Jing, Shu Jiayu, Dong Qiang. Research progress in platelet-rich fibrin in stomatology[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 441-446.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
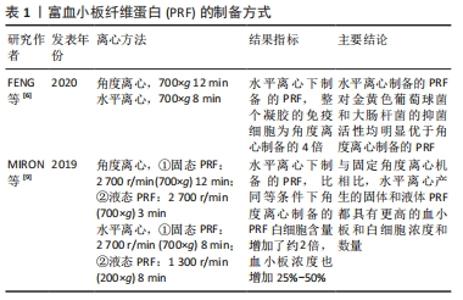
2.1 PRF的制备 通过采集患者自体血,经过离心(300 r/min 10 min或者225 r/min 12 min)后获得3层结构,红色凝胶状的红细胞底层,淡黄色凝胶状的中间层以及淡黄色澄清液体状的血浆上层,去除上清及红细胞层,即可获到中间的PRF凝胶层,大部分的白细胞和血小板聚集在此层。其具有一定的韧性、可塑性及弹性,因此,可根据临床需求,用无菌纱布或专用金属器械盒压制成纤维蛋白膜,或者与其他支架材料复合使用,亦或与骨替代材料混合[3]。 与富血小板血浆相比,PRF的制备过程中不添加任何抗凝剂,避免了免疫排斥反应、交叉感染等风险。正因如此,PRF的制备成功取决于从采集自体血到离心时的时间间隔,因为没有添加抗凝剂,血液一旦接触管壁,就立刻开始凝固,为防止离心后不能形成完好的纤维蛋白膜,所以这个间隔越短越好[8]。 目前制备PRF大多用角度离心法,大部分的白细胞以及血小板都分布在黄色凝胶上层与红细胞凝胶状底层的交界处约1 mm,然而,FENG等[6]通过水平离心法制备的PRF中所含的免疫细胞数量为原方法的4倍,并且白细胞数量也显著增多,表明了水平离心制作的PRF或许具有更强的抗菌能力。此外,MIRON等[9]应用水平离心制备的固态PRF(700×g 8 min)和液态PRF(200×g 8 min),与角度离心制备的固态PRF(700×g 12 min)、高级PRF(200×g 8 min)以及液态PRF(700×g 3 min)、可注射的PRF(60×g 3 min)相比,通过连续移液法测定,前二者白细胞含量增加了约2倍,血小板浓度也增加了25%-50%,表明水平离心制备的PRF可能具有更好的止血、抗菌能力,或许是未来血液衍生物应用的一个方向。PRF的制备方式见表1。 "
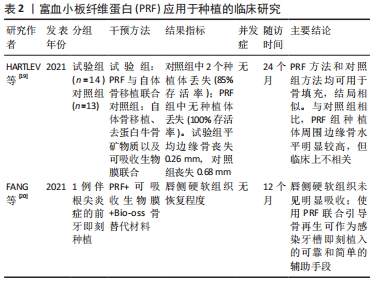
2.2 PRF的生物学特性 HERMIDA-NOGUEIRA等[2]用一项体外培养实验获得了PRF释放的蛋白组成,以便更确切地了解PRF在伤口愈合中的影响,在培养的第3天,PRF分泌组中鉴定出705种蛋白,并且发现多数生长因子在第3天富集,且随着时间推移数量减少,而在第7天,由单核细胞分化而成的巨噬细胞含量有所增加。巨噬细胞已被证明是组织再生、伤口愈合和预防感染的关键因素,此外它们还具有抗菌作用,能够减少术后细菌污染[1]。 有研究表明,天然的血凝块为立体的三维结构,且疏松多孔[10-12];而在电镜扫描下,PRF的纤维蛋白结构类似于天然的血凝块[3],疏松多孔的同时具有一定的弹性,该结构能够使纤维蛋白与生长因子产生化学键的结合[13],使生长因子能够有效储存在PRF凝胶内发挥作用,减缓了生长因子的流失,增强了其生物学功能。立体的三维结构同时可以起到支架的作用,为组织细胞及干细胞的增殖分化提供所需的场所,其网格状结构能够保证血小板不被立即活化[14-15]。随着纤维蛋白的溶解逐渐脱颗粒,缓慢释放生长因子,包括转化生长因子β、血管内皮生长因子、血小板源性生长因子及胰岛素样生长因子,这些生长因子相辅相成、协同作用,调控成骨细胞、成纤维细胞以及干细胞的增殖分化,促进软硬组织的愈合[1];此外,有研究表明,PRF中还含有白细胞介素1β、白细胞介素4、白细胞介素6和肿瘤坏死因子α,在组织愈合中起着主要的炎症调节、抗感染和促进组织新生的作用[16]。 有研究表明PRF有着成血管能力,其诱导血管生成过程的体外关键步骤:内皮增殖、迁移和血管化,RATAJCZAK等[5]用抗体列阵充分表明了PRF在体外血管化的潜能。并且发现了高水平的CXC趋化因子受体2(CXCR-2)配体和表皮生长因子(EGF),二者在成血管中起到重要作用;同时,利用绒毛尿囊膜试验清楚地证明PRF能够在体内诱导血管形成。 2.3 PRF在口腔种植领域的应用 近年来,随着口腔种植技术的飞速发展,种植固定义齿已经成为了牙列缺损和牙列缺失的常规修复手段。但是由于部分患者口腔剩余牙槽骨骨量不足,成骨能力低下,导致此类患者种植成功率相对较低。而随着PRF概念的提出以及成功制备,越来越多的学者将其运用到口腔种植中,提高了种植成功率,并扩展了种植治疗适应证[17]。KARGARPOUR等[18]的体外实验表明,PRF在一定程度上能够抑制破骨细胞的生成,这或许是PRF能够保存牙槽骨宽度和高度的方式之一。种植成功的标准之一是获得良好的“骨结合”,PRF在骨结合早期阶段增加了种植体的稳定性,从而在第1个月时种植体能够获得更高的初期稳定性[17]。一项临床随机对照试验比较了PRF与自体骨移植联合使用(试验组,n=14)与自体骨移植、去蛋白牛骨矿物质以及可吸收生物膜联合使用(对照组,n=13)的效果,在最终冠修复24个月后的临床以及影像学随访中,显示试验组种植体存活率达100%,且种植体周边缘骨水平明显较高[19]。然而,该研究样本量较小,并且种植位点包括了切牙、尖牙以及前磨牙,因此应谨慎解释上述结果。一篇前牙美学区残根伴严重根尖周炎的即刻种植个例,在随访1年后未见唇侧骨组织明显吸收以及软组织塌陷,患者自觉美学效果良好[20]。表明了多层次PRF的使用不仅为维持稳定空间提供了良好的力学性能,而且在促进组织再生和预防感染方面具有良好的生物学功能。PRF应用于种植的临床研究见表2。 "
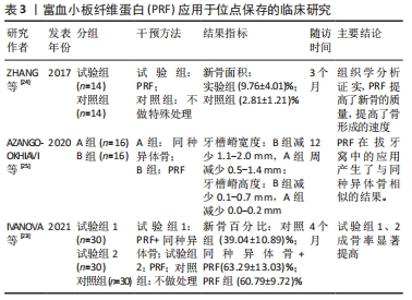
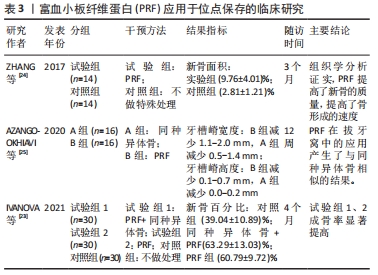
2.3.1 PRF在位点保存中的应用 有研究表明,拔牙后6个月的自然愈合过程中,牙槽窝颊侧的垂直高度将减小11%-22%,水平宽度减小将更明显,为29%-63%[21]。一项前瞻性研究指出,拔牙后12个月内牙槽嵴宽度减少50%,为5-7 mm,同时发现大约2/3的变化发生在拔牙后的前3个月内[22],再加上患者很难保证口腔健康以及其他各次级因素,拔牙后牙槽窝的良好愈合很难得到保障,这更加剧了牙种植手术难度及失败风险。因此,在位点保存术中,应用PRF的特性能够促血管化、利于新生骨组织的形成,同时其抗炎抗感染的作用有利于组织愈合。一项临床随机对照试验指出,在拔牙后4个月,对照组(血凝块充填拔牙窝)新生骨百分比为39.04%,远远小于试验组(PRF与同种异体骨联合使用充填拔牙窝以及单独使用PRF充填拔牙窝)的新生骨百分比63.29%、60.79%[23],提示了PRF能够有效促进成骨效果,从而良好地保存牙槽骨的形态。此外,另一项临床试验将28例患者分为试验组与对照组,前者患者在拔除患牙后对拔牙窝不做处理,后者填入PRF;经过3个月的随访调查,经CBCT分析表明牙槽嵴的宽度以及高度的平均值,二者无统计学差异,然而组织学分析表明与对照组相比,PRF组的新骨面积显著增大,这可能是由于生长因子的浓度,PRF能够提高新骨的质量和骨形成率[24]。这些结果表明,PRF单独运用于位点保存术中因为其具有大量生长因子的特性,可以刺激新骨形成[23-24]。一项临床试验对比了PRF和同种异体骨分别单独应用于位点保存术中的疗效,将32例患者分为2组,一组患者在拔除患牙后使用同种异体骨填入拔牙窝,另一组填入PRF,分别在拔牙后即刻、12周后对牙槽骨高度及宽度进行测量,结果显示二者无统计学意义[25],表明PRF对于牙槽骨形态的保存起到了积极作用,且方便、快捷。 然而,2篇Meta分析的研究证据表明单独使用PRF来保存牙槽骨时并不会带来有利的效果[26-27]。但是,一篇系统综述提出单独使用PRF进行位点保存时牙槽骨水平距离的减少相对于自然愈合有所降低[28],由于对水平牙槽骨的评价方式不同,因此该结论不具备共性。PRF应用于位点保存的临床研究见表3。 "

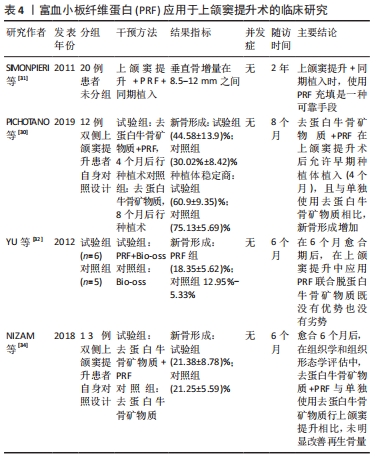
2.3.2 PRF在上颌窦提升术中的应用 2004年,CHOUKROUN等[29]首次将PRF与冻干同种异体骨联合运用于上颌窦提升术,获得了良好的愈合效果,提出PRF与冻干同种异体骨的混合物应用在上颌窦提升术中,可使种植术前的愈合时间减少到4个月。在上颌窦提升术应用PRF联合去蛋白牛骨矿物质后,PICHOTANO等[30]评估了早期种植体的稳定性,发现4个月后就能够进行牙种植术,与单独使用去蛋白牛骨矿物质愈合的8个月后的种植术相比,前者的新骨形成更明显。SIMONPIERI等[31]对20例上颌窦外提升单纯植入PRF后同期行种植术的患者,进行了长达6年的随访后发现,种植体的存活率为100%,上颌窦窦底骨高度平均提升了8.5-12 mm。有学者通过对PRF添加到骨替代材料中行上颌窦外提升术的研究发现,实验组的新生骨是对照组(骨替代材料)的1.4倍[32];此外,另一项研究显示,用PRF经上颌窦内提的患者,组织学观察到更快的骨愈合[33]。但是,也有证据提示,在上颌窦提升术中PRF的研究结果不确定。NIZAM等[34]探讨了PRF与去蛋白牛骨矿物质在上颌窦提升术中的联合使用,通过对13例患者26个手术位点的分析、随访,结果表明,在去蛋白牛骨矿物质中添加PRF并没有提高再生骨的数量。一项临床研究表明,在上颌窦大穿孔(>15 mm)的情况下应用PRF修补并同期植入种植体,且在术后12个月通过放射学检查,发现上颌窦底平均高度增加了6.43 mm,最大达9 mm;通过组织学评估,获得的平均活骨量为52.30%;而micro-CT 3D中的骨体积/组织体积比的平均值为50.32%[35]。然而,该研究样本量较少,上述的统计分析不具备代表性。一篇循证临床疗效表明,在使用脱蛋白牛骨矿物质与PRF组合的 5 项研究中,60%的人认为没有显著影响;只有1项研究使用PRF作为上颌窦提升唯一的移植材料并报告了积极的效果[36];但由于纳入研究的异质性,该综述无法进行适当的系统荟萃分析,因此,还需要进行大量的随机试验来证实且保证PRF在上颌窦提升术中的积极作用。PRF应用于上颌窦提升术的临床研究见表4。"
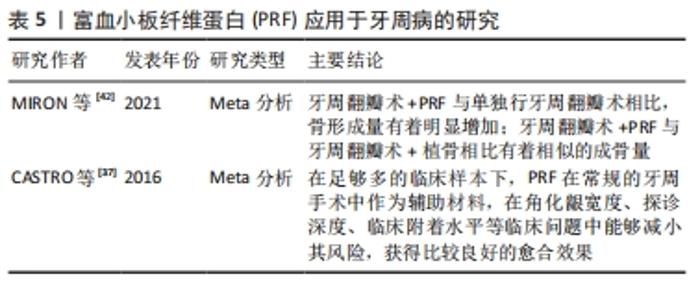
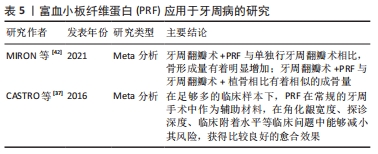
2.4 PRF在牙周病学中的应用 牙周疾病是口腔最常见疾病,以牙龈萎缩、牙周出血、牙齿松动脱落为主要病症,不仅危害口腔健康,同时还影响身心健康。由于牙周组织的复杂性,损伤后的牙周组织难以修复。目前,临床上主要的治疗手段是控制感染、去除病因,但很难重新恢复牙周组织。而牙龈萎缩更是牙周美学修复的一个焦点问题。一篇系统综述就PRF在牙周疾病中的应用做详细的分析描述,在足够多的临床样本下,PRF在常规的牙周手术中作为辅助材料,在角化龈宽度、探诊深度、临床附着水平等临床问题中能够减小其风险,获得比较良好的愈合效果[37]。此外,也有其他证据表明,PRF能够抑制牙龈卟啉单胞菌的生长[38],并且可以诱导牙龈成纤维细胞和牙周结缔组织成纤维细胞增殖,作为创口愈合与修复的潜在材料,同时该证据指出这种修复、愈合方式并不会受到患者牙周状况的影响[39]。KOUR等[7]通过一项体外实验证实了富血小板血浆、PRF、可注射的PRF三种血液衍生物对牙龈卟啉单胞菌和伴放线聚集杆菌显示出一定的抗菌活性。然而,可注射的PRF和PRF的制备不需要任何添加剂和抗凝剂,具有完全自体的优势。DEBNATH等[40]的一项临床试验指出,对于Miller’s Ⅰ类或Ⅱ类牙龈退缩的患者,冠向复位瓣与PRF联合使用后,经过3-6个月的追踪观察,探针深度、牙周附着水平以及角化龈宽度均有很大程度改善。这提示:与传统结缔组织移植相比,该方法避免开辟第二术区,患者更容易接受,不适度更小。 一篇叙述性综述比较了富血小板血浆、PRF以及浓缩生长因子在牙周组织中再生的作用,指出富血小板血浆主要用于硬、软组织手术,PRF主要用于牙龈退缩和分叉、骨内缺损的治疗,浓缩生长因子主要用于骨再生[41]。然而,由于该文章是叙事性的回顾,因此解释时应谨慎。一篇系统综述指出PRF单独使用或与骨替代材料联合使用时,牙周组织愈后的探诊深度、临床附着水平以及放射学骨充填均有明显改善[42]。一项犬的动物实验表明PRF在牙周治疗中加速了创口愈合,减少了炎症的发生[43]。但是仍需大量的随机对照试验才能够保证临床上的积极效果。PRF应用于牙周病的研究见表5。 "
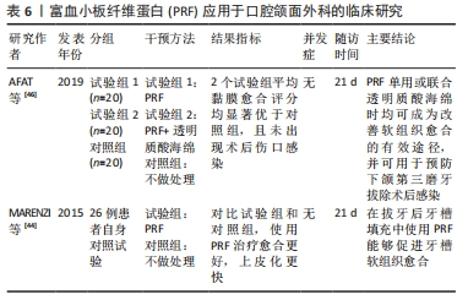
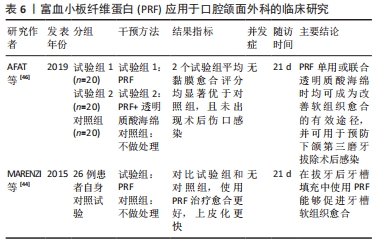
2.5 PRF在口腔颌面外科中的应用 由于PRF具有促进软硬组织愈合的特性,其在口腔颌面外科的应用也越来越广泛。一项前瞻性自身对照试验表明,与拔牙窝自然愈合相比,在拔牙术后一侧拔牙窝填入PRF,在术后前7 d愈合速度更快,拔牙窝闭和更迅速,上皮化更明显,局部炎症反应也相对减少[44]。近年来,对于PRF在下颌第三磨牙拔除术后牙槽窝愈合影响的相关报道越来越多。一篇Meta分析认为,PRF对下颌阻生第三磨牙拔除后的骨愈合无积极作用[45]。然而,由于纳入研究的异质性大和样本量小,上述结论应谨慎解释,该文作者建议进行进一步设计良好的前瞻性随机对照试验,以进一步解释PRF对下颌第三磨牙拔除术后的影响。同时,一项前瞻性临床研究指出,单独使用PRF或与透明质酸联合使用均可有效促进软组织愈合,可预防下颌第三磨牙拔除术后牙槽骨炎及感染[46]。然而,该研究样本量较小,不足以评价术后并发症,因此需要更大的样本量行进一步研究。SINGH等[47]进行了一项为期3个月的临床试验,20例患者拔除双侧下颌第三磨牙后,一侧拔牙窝填入PRF,另一侧作为对照组,结果研究侧疼痛较对照组减轻,软组织愈合较好;同时3个月后经放射学评估骨密度显示,在放置PRF部位计算出的灰度值明显高于对照组部位骨密度平均基线值。该试验表明自体PRF具有生物相容性,能够改善软组织愈合,并且在拔牙后有助于骨再生以及骨密度增加,然而仍需要大量的临床样本来证实这一结论的可靠性。除此之外,有研究报道使用了PRF治疗上颌后牙区的大囊肿,在囊肿术后同期放入PRF与骨替代材料混合物,取得了良好的效果,并在6个月后进行了3个牙位的种植体植入术,最终获得了良好的修复效果[48]。尽管该病例报道存在一定局限性,但该文作者认为,PRF在口腔外科手术中的应用对于骨愈合和组织再生方面起到了积极作用。PRF应用于口腔颌面外科的临床研究见表6。"
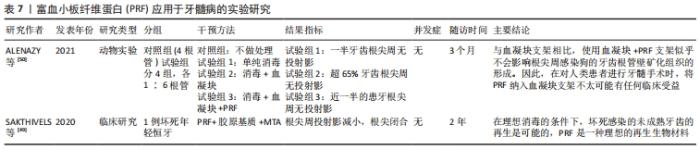
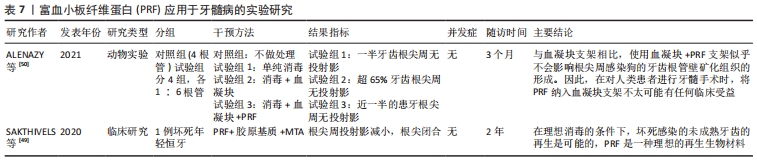
2.6 PRF在牙髓病学中的应用 由于PRF有一定的支架作用,因此有学者将其运用在牙髓再生,期望获得良好的临床疗效。一例病例报道了PRF与胶原基质联合运用于一颗因感染导致牙髓坏死而根尖孔未发育完全的中切牙,12个月随访时进行影像学检查,显示根尖闭合约1.5 mm,2年后随访患牙功能正常,影像学资料显示根尖直径1 mm[49]。但是,该报道因其局限性不具有代表性,因此还需要大量的随机临床试验来进一步研究。一项犬的动物实验指出PRF与血凝块的混合支架,与单独血凝块支架相比,似乎不会影响沿根管壁矿化组织的形成[50]。因此,在临床上进行牙髓再生手术时,在血凝块内加入PRF似乎不会取得良好的临床收益。同时,另外一篇关于牙髓再生研究的文献也支持在未成熟坏死牙齿中,PRF在促进根管壁增厚或诱导根尖发育方面,与血凝块相比缺乏明显的优势[51]。因此,在血液衍生物运用于牙髓再生这一方向还需要行进一步的研究。PRF应用于牙髓病的实验研究见表7。"

2.7 PRF与术后疼痛的关系 无论在种植术后或者牙拔除术后,疼痛都是评价的指标之一。近年来,有相关研究报道术中填入PRF能够减轻疼痛的感觉。TEMMERMAN等[52]研究表明,种植术中使用PRF,术后3-5 d患者疼痛的感觉显著减轻;MARENZI等[44]的研究显示,在拔牙窝内填入PRF,疼痛情况较对照组有明显减轻。大量研究评估了PRF对下颌阻生第三磨牙拔除术后疼痛的影响,结论显示,使用PRF后术区疼痛、水肿等症状明显减轻[45-47,53]。但是这些研究的不足之处在于,患者是在知情的情况下得到的结果,同时仍缺少循证证据证明,种植术中PRF的使用能够有效减轻患者的疼痛感。因此,对于PRF能够减轻患者疼痛的研究报告需要谨慎对待。"

| [1] MIRON RJ, FUJIOKA-KOBAYASHI M, BISHARA M, et al. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng Part B Rev. 2017;23:83-99. [2] HERMIDA-NOGUEIRA L, BARRACHINA MN, MORÁN LA, et al. Deciphering the secretome of leukocyte-platelet rich fibrin: towards a better understanding of its wound healing properties. Sci Rep. 2020;10:14571. [3] DOHAN EHRENFEST DM, DEL CORSO M, DISS A, et al. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546-555 [4] HUANG Y, BORNSTEIN MM, LAMBRICHTS I, et al. Platelet-rich plasma for regeneration of neural feedback pathways around dental implants: a concise review and outlook on future possibilities. Int J Oral Sci. 2017;9:1-9. [5] RATAJCZAK J, VANGANSEWINKEL T, GERVOIS P, et al. Angiogenic Properties of ‘Leukocyte- and Platelet-Rich Fibrin’. Sci Rep. 2018;8:14632. [6] FENG M, WANG Y, ZHANG P, et al. Antibacterial effects of platelet-rich fibrin produced by horizontal centrifugation. Int J Oral Sci. 2020;12:32. [7] KOUR P, PUDAKALKATTI PS, VAS AM, et al. Porphyromonas gingivalisComparative Evaluation of Antimicrobial Efficacy of Platelet-rich Plasma, Platelet-rich Fibrin, and Injectable Platelet-rich Fibrin on the Standard Strains of and Aggregatibacter actinomycetemcomitans. Contemp Clin Dent. 2018;9:S325-S330. [8] DOHAN DM, CHOUKROUN J, DISS A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-44. [9] Miron RJ, Chai J, Zheng S, et al. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A. 2019;107:2257-2271. [10] MOSESSON MW, SIEBENLIST KR, MEH DA. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11-30. [11] Dohan DM, MD C, Charrier JB. Cytotoxicity analyses of Choukroun’s platelet-rich fibrin (PRF) on a wide range of human cells: The answer to a commercial controversy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(5):587-593. [12] HO W, TAWIL B, DUNN JC, et al. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure.Tissue Eng. 2006;12:1587-1595. [13] HOLDERFIELD MT, HUGHES CC. Crosstalk between vascular endothelial growth factor, notch,and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637-652. [14] MARX RE, CARLSON ER, EICHSTAEDT RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638-646. [15] DOHAN DM, CHOUKROUN J, DISS A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45-50. [16] DOHAN DM, CHOUKROUN J, DISS A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates?Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:e51-55. [17] STRAUSS FJ, STAHLI A, GRUBER R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin Oral Implants Res. 2018;29 Suppl 18(Suppl Suppl 18):6-19. [18] KARGARPOUR Z, NASIRZADE J, STRAUSS FJ, et al. Platelet-rich fibrin suppresses in vitro osteoclastogenesis. J Periodontol. 2020;91:413-421. [19] Hartlev J, Schou S, Isidor F, et al. A clinical and radiographic study of implants placed in autogenous bone grafts covered by either a platelet-rich fibrin membrane or deproteinised bovine bone mineral and a collagen membrane: a pilot randomised controlled clinical trial with a 2-year follow-up. Int J Implant Dent. 2021;7:8. [20] Fang J, Xin X, Li W, et al. Immediate implant placement in combination with platelet rich-fibrin into extraction sites with periapical infection in the esthetic zone: A case report and review of literature. World J Clin Cases. 2021;9:960-969. [21] JUNG RE, IOANNIDIS A, HAMMERLE CH, et al. Alveolar ridge preservation in the esthetic zone. Periodontol 2000. 2018;77:165-175. [22] SCHROPP L, WENZEL A, KOSTOPOULOS L, et al. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23:313-323. [23] Ivanova V, Chenchev I, Zlatev S, et al. Comparison Study of the Histomorphometric Results after Socket Preservation with PRF and Allograft Used for Socket Preservation—Randomized Controlled Trials. Int J Environ Res Public Health. 2021;18(14):7451. [24] Zhang Y, Ruan Z, Shen M, et al. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp Ther Med. 2018;15:2277-2286. [25] Azangookhiavi H, Ghodsi S, Jalil F, et al. Comparison of the Efficacy of Platelet-Rich Fibrin and Bone Allograft for Alveolar Ridge Preservation after Tooth Extraction: A Clinical Trial. Front Dent. 2020;17:1-6 [26] LIN CY, CHEN Z, PAN WL, et al. Effect of Platelet-Rich Fibrin on Ridge Preservation in Perspective of Bone Healing: A Systematic Review and Meta-analysis. Int J Oral Maxillofac Implants. 2019;34:845-854. [27] AREEWONG K, CHANTARAMUNGKON M, KHONGKHUNTHIAN P. Platelet-rich fibrin to preserve alveolar bone sockets following tooth extraction: A randomized controlled trial. Clin Implant Dent Relat Res. 2019;21:1156-1163. [28] PAN J, XU Q,HOU J, et al. Effect of platelet-rich fibrin on alveolar ridge preservation: A systematic review. J Am Dent Assoc. 2019;150:766-778. [29] CHOUKROUN J, DISS A, SIMONPIERI A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299-303. [30] PICHOTANO EC, MOLON RS, SOUZA RV, et al. Evaluation of L-PRF combined with deproteinized bovine bone mineral for early implant placement after maxillary sinus augmentation: A randomized clinical trial. Clin Implant Dent Relat Res. 2019;21:253-262. [31] SIMONPIERI A, CHOUKROUN J, DEL CORSO M, et al. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent. 2011;20(1):2-12. [32] Yu Z, Tangl S, Huber CD, et al. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J Craniomaxillofac Surg. 2012;40(4):321-328. [33] Tatullo M, Marrelli M, Cassetta M, et al. Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci. 2012;9:872-880. [34] NIZAM N, EREN G, AKCALI A, et al. Maxillary sinus augmentation with leukocyte and platelet-rich fibrin and deproteinized bovine bone mineral: A split-mouth histological and histomorphometric study. Clin Oral Implants Res. 2018;29:67-75. [35] Barbu HM, Iancu SA, Hancu V, et al. PRF-Solution in Large Sinus Membrane Perforation with Simultaneous Implant Placement-Micro CT and Histological Analysis. Membranes. 2021;11(6):438. [36] Damsaz M, Castagnoli CZ, Eshghpour M, et al. Evidence-Based Clinical Efficacy of Leukocyte and Platelet-Rich Fibrin in Maxillary Sinus Floor Lift, Graft and Surgical Augmentation Procedures. Front Surg. 2020;7:537138. [37] CASTRO AB, MESCHI N, TEMMERMAN A, et al. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol. 2017;44:67-82. [38] CASTRO AB, HERRERO ER, SLOMKA V, et al. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci Rep. 2019;9:8188. [39] GOEL A, WINDOR LJ, GREGORY RL, et al. Effects of platelet-rich fibrin on human gingival and periodontal ligament fibroblast proliferation from chronic periodontitis versus periodontally healthy subjects. Clin Exp Dent Res. 2021;7(4): 436-442. [40] DEBNATH K, CHATTERJEE AA. Evaluation of periosteum eversion and coronally advanced flap techniques in the treatment of isolated Miller’s Class I/II gingival recession: A comparative clinical study. J Indian Soc Periodontol. 2018;22:140-149. [41] Mijiritsky E, Assaf HD, Peleg O, et al. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation-A Narrative Review. Biology. 2021;10(4):317. [42] Miron RJ, Moraschini V, Fujioka-Kobayashi M, et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: a systematic review and meta-analysis. Clin Oral Investig. 2021;25:2461-2478. [43] KORNSUTHISOPON C, PIRARAT N, OSATHANON T, et al. Autologous platelet-rich fibrin stimulates canine periodontal regeneration. Sci Rep. 2020;10:1850. [44] Marenzi G, Riccitiello F, Tia M, et al. Influence of Leukocyte- and Platelet-Rich Fibrin (L-PRF) in the Healing of Simple Postextraction Sockets: A Split-Mouth Study. Biomed Res Int. 2015;2015:369273. [45] Al-Hamed FS, Tawfik MA, Abdelfadil E, et al. Efficacy of Platelet-Rich Fibrin After Mandibular Third Molar Extraction: A Systematic Review and Meta-Analysis. J Oral Maxillofac Surg. 2017;75:1124-1135. [46] Afat IM, Akdoğan ET, Gönül O. Effects of leukocyte- and platelet-rich fibrin alone and combined with hyaluronic acid on early soft tissue healing after surgical extraction of impacted mandibular third molars: A prospective clinical study. J Craniomaxillofac Surg. 2019;47:280-286. [47] SINGH A, KOHLI M, GUPTA N. latelet rich fibrin: a novel approach for osseous regeneration.J Maxillofac Oral Surg. 2012;11:430-434. [48] Mauceri R, Murgia D, Cicero O, et al. Leucocyte- and Platelet-Rich Fibrin Block: Its Use for the Treatment of a Large Cyst with Implant-Based Rehabilitation. Medicina (Kaunas). 2021;57(2):180. [49] Sakthivel S, Gayathri V, Anirudhan S, et al. Platelet-rich fibrin and collagen matrix for the regeneration of infected necrotic immature teeth. J Clin Transl Res. 2020;6:1-5. [50] Alenazy MS, Al-Nazhan S, Mosadomi HA. Histologic, Radiographic, and Micro-Computed Tomography Evaluation of Experimentally Enlarged Root Apices in Dog Teeth with Apical Periodontitis after Regenerative Treatment. Curr Ther Res Clin Exp. 2021;94:100620. [51] Riaz A, Shah FA. Regenerating the Pulp-Dentine Complex Using Autologous Platelet Concentrates: A Critical Appraisal of the Current Histological Evidence. Tissue Eng Regen Med. 2021;18:37-48. [52] Temmerman A, Vandessel J, Castro A, et al. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol. 2016;43:990-999. [53] Afat IM, Akdoğan ET, Gönül O. Effects of Leukocyte- and Platelet-Rich Fibrin Alone and Combined With Hyaluronic Acid on Pain, Edema, and Trismus After Surgical Extraction of Impacted Mandibular Third Molars. J Oral Maxillofac Surg. 2018;76:926-932. |
| [1] | Yu Weijie, Liu Aifeng, Chen Jixin, Guo Tianci, Jia Yizhen, Feng Huichuan, Yang Jialin. Advantages and application strategies of machine learning in diagnosis and treatment of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1426-1435. |
| [2] | Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300. |
| [3] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [4] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [5] | Li Jiaqi, Huang Yuanli, Li Yan, Wang Chunren, Han Qianqian. Mechanism and influencing factors in molecular weight degradation of non-cross-linked hyaluronic acid [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 747-752. |
| [6] | Xu Rong, Wang Haojie, Geng Mengxiang, Meng Kai, Wang Hui, Zhang Keqin, Zhao Huijing. Research advance in preparation and functional modification of porous polytetrafluoroethylene artificial blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 759-765. |
| [7] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| [8] | Liu Chuang, Shan Shuo, Yu Tengbo, Zhou Huan, Yang Lei. Advantages, discomfort and challenges of clinical application of orthopedic hemostatic materials [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 795-803. |
| [9] | Zhang Ming, Wang Bin, Jia Fan, Chen Jie, Tang Wei. Application of brain-computer interface technology based on electroencephalogram in upper limb motor function rehabilitation of stroke patients [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 581-586. |
| [10] | He Yuanjie, Chen Yuheng, Zhao Yongchao, Wang Zhenglong. Progress in epigenetic regulation of vascular smooth muscle cell remodeling in the occurrence and development of aortic aneurysms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 602-608. |
| [11] | Ma Sicong, Chen Jing, Li Yunqing. Functions and roles of connective tissue growth factor in nervous systems [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 615-620. |
| [12] | Yan Binghan, Li Zhichao, Su Hui, Xue Haipeng, Xu Zhanwang, Tan Guoqing. Mechanisms of traditional Chinese medicine monomers in the treatment of osteoarthritis by targeting autophagy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 627-632. |
| [13] | Zhi Liang, Wang Yulong, Zhang Qingfang, Hong Yaqing, Ke Meihua, Liu Quanquan, Long Jianjun. Propulsion deficits in hemiplegic gait of stroke patients [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5709-5715. |
| [14] | Wang Wenchi, Wu Ruiqi, Huang Jierong, Zhu Lifeng, Cui Xianqin, Li Dongzong, Chen Wenhui, Lin Chunting, Cui wei. Molecular mechanism of naringin in prevention and treatment of osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5528-5535. |
| [15] | Li Qi, Gao Mingwei, Li Shihao, Chu Xiaolei, Li Yajie, Ding Ning, Liu Minqi. Rate of force development and its relationship with functional performance in patients after anterior cruciate ligament reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5536-5543. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||