Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (16): 2597-2604.doi: 10.3969/j.issn.2095-4344.3081
Previous Articles Next Articles
Problems and trends of technique and clinical application of metallic biomaterials prepared by three-dimensional printing technology
Ji Qi, Yu Zhengwen, Zhang Jian
- School of Stomatology, Zunyi Medical University, Zunyi 563099, Guizhou Province, China
-
Received:2020-06-17Revised:2020-06-20Accepted:2020-07-30Online:2021-06-08Published:2021-01-07 -
Contact:Zhang Jian, Associate professor, Master’s supervisor, School of Stomatology, Zunyi Medical University, Zunyi 563099, Guizhou Province, China -
About author:Ji Qi, Master candidate, School of Stomatology, Zunyi Medical University, Zunyi 563099, Guizhou Province, China -
Supported by:a grant from The Sixth Batch of Talent Base Construction Project of Organization Department of Guizhou Provincial Party Committee, No. RCJD2018-9 (to ZJ and YZW); the Youth Science and Technology Talent Growth Project of Guizhou Provincial Department of Education, No. KY [2016]207 (to YZW); the Joint Research and Development Fund Project of Zunyi Science and Technology Bureau and Zunyi Medical University, No. (2016)45 (to YZW)
CLC Number:
Cite this article
Ji Qi, Yu Zhengwen, Zhang Jian. Problems and trends of technique and clinical application of metallic biomaterials prepared by three-dimensional printing technology[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2597-2604.
share this article

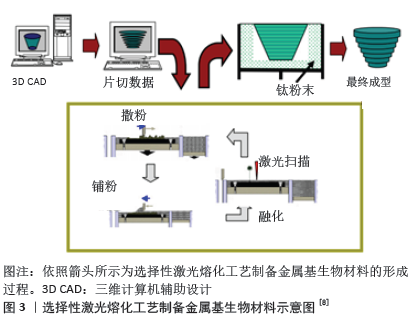
2.1 生物医学应用的金属3D打印技术 在生物医学应用中,按金属3D打印工艺原理不同分为2大类:粉末床选区熔融和定向能量沉积两大类别。粉末床选区熔融系统分为选择性激光熔化、直接金属激光烧结、选择性激光烧结和电子束熔化技术[5-6]。定向能量沉积工艺包括激光金属沉积和激光工程网成形[7]。以选择性激光熔化技术为例简要叙述金属3D打印基本工作原理,见图3:通过CAD或CT给定要制造物体的三维模型,将模型分解为二维切片,并将得到的数据转化为数控工作台的运动轨迹,在真空或惰性气体保护室中将一薄层金属粉末均匀地铺在建筑平台上,根据预定运动轨迹利用高功率能量器将金属粉末选择性地熔化,当一层的金属粉末熔化完成时建筑平台降低预定距离(通常为20-100 mm),并且将下一层粉末沉积在平台上,然后重复该过程直到完成所需的零件为止[8]。"

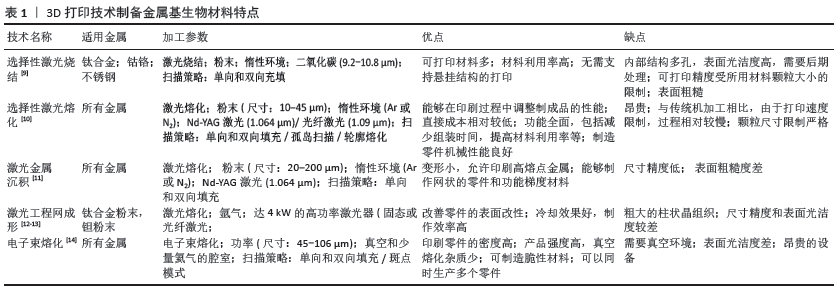
目前,通过金属3D打印能够批量生产具有复杂几何形状和内部结构的金属植入物,以及满足特定患者需求的定制医疗植入物生产;面对诸多的金属打印技术,需根据要制造的零件的复杂性或设计注意事项不同而选择适宜的增材制造工艺。该节列举当前常用几种金属3D打印技术的特点及优缺点,见表1[9-14]。 2.2 金属基材料研究进展 金属生物材料应具有以下基本特征:优异的生物相容性;高耐腐蚀性;合适的机械性能;高耐磨性;骨整合[15];具体的设计和选择取决于其特定的医学应用,例如:用于整形外科或牙科应用的生物材料应具有较高的机械刚度和缓慢的生物降解速率。在该节中将介绍几种研究较为深入的金属基生物材料当前的研究进展。 2.2.1 钛基金属材料 钛(Ti)是3D打印中使用最广泛的金属材料,具有高的机械强度、低弹性模量、低密度、高耐蚀性和良好的生物相容性等优点,应用于负重植入物,例如膝关节和全髋关节置换。尽管与其他金属生物材料相比钛的成本更高,但凭借独特的性能使其能够在长期使用中达到更高的成本效益[16-17]。目前,在90%的传统骨科植入物中首选的生物金属是Ti-6Al-4V(TC4)和商业纯钛(CP-Ti)[18]。 植入物的临床成功与否不仅取决于出色的耐腐蚀性和良好的组织反应,还取决于其功能设计[19]。传统致密的金属植入物由于其密度、刚度和弹性模量比骨组织差异大,容易引起应力屏蔽效应,导致植入物无菌性松动[20]。由钛合金粉末制备的多孔金属植入物可以改变孔隙梯度孔径、不同的孔及孔之间的三维分布设计,改善金属植入物的弹性模量[21]。此外研究表明,多孔钛合金植入物具有良好的骨向内生长能力,支持人体骨细胞的生长,增大植入物接触表面积,并在植入物和骨骼之间形成牢固的扭转锁,显著提高植入物的成功率[22]。多孔结构的另一个显著特征是其高比表面积,可以通过表面改性改善3D打印多孔医用金属植入物的表面生物活性、耐磨性和抗凝性,进一步提高稳定性和长期有效性。目前,选择性激光融化和电子束熔化是3D打印钛合金最常用的技术[23]。临床组织学实验表明,3D打印植入物比常规植入物具有更快的骨整合率[24]。上述对钛基材料的研究大多集中在微观结构和机械特性的改进上,因此在生物相容性方面仍有很大的验证空间。有学者通过体内实验研究选择性激光融化和电子束熔化制备钛合金植入物的生物相容性[25]。PALMQUIST等[26]将电子束熔化打印的多孔和无孔致密TC4植入物分别植入绵羊两侧的股骨及背部皮下评估其骨整合能力,结果显示,在植入26周后多孔和无孔植入物表现出良好的软组织生物相容性和高度的骨整合度,且多孔植入物与骨骼的接触率更高,最高可达57%,表明电子束熔化制备的多孔TC4植入物在骨骼和软组织修复中具有一定的发展前景。除了这些研究结果外,近年来还报道了一些成功植入3D打印钛植入物的手术,这些报告表明个性化的医疗植入物可减少手术和住院时间,从而降低总体医疗成本,证实了3D打印钛基金属材料在临床应用中具有巨大的潜能。 2.2.2 钴铬合金 钴铬合金是主要由钴(Co)和铬(Cr)组成的高温合金,它们具有出色的耐腐蚀性、机械性能和生物相容性,是理想的承重植入物[27]。钴铬合金最早用于人造关节,现在已被广泛用于口腔科,由于它们不含镍和锑等有害元素,因此3D打印钴铬合金烤瓷牙已成为非贵金属烤瓷装置的首选[28]。此外,对选择性激光熔化制备牙科,钴铬合金的微观结构、机械性能、腐蚀性和生物相容性进行了广泛研究[29],这些研究表明,3D打印的钴铬合金牙科植入物可以准确地模仿牙槽骨的自然结构,且在表面覆盖密集的贯通孔隙以促进成骨细胞的再生。HAZLEHURST等[30]使用选择性激光熔化来制造孔隙率范围为25%-95%的钴铬钼(CoCrMo)多孔结构,这些多孔结构的刚度和强度类似于人股骨的皮质骨和松质骨,证明多孔结构可以减少植入物75%-80%的有效弹性模量,使应力屏蔽最小化,增强长期的骨整合稳定性[31]。但是作为承重植入物,钴铬合金放置在人体内部时仍然存在磨损和腐蚀问题,而由此产生的金属离子释放将导致各种医学并发症,致植入物失败。SAHASRABUDHE等[32]通过激光工程网成形技术制备了含3%CaP的钴铬钼合金,发现样品磨损率仅为纯CoCrMo合金的1/3,且Co2+和Cr2+的释放量也减少了4倍。 2.2.3 镁基金属 相对于不可降解的钛和不锈钢等惰性生物金属,镁与人体骨骼具有最佳的生物力学相容性(因为其密度相近)。此外,镁可以被人体吸收并以镁离子的形式释放,增强成骨细胞的增殖和分化,从而促进骨骼的生长和愈合[33]。除了诸如骨螺钉和骨板之类的医疗设备之外,镁合金还可以制成可降解的心血管支架[34]。JAUER等[35]通过选择性激光熔化成功地制造了具有互连孔隙度的WE43(Mg-RE合金)支架状结构。此后,LI等[36]报道了由选择性激光熔化制造的排序且多孔联通的可生物降解WE43支架,其在4周后仅降解约20%的体积,并且仍保持了小梁骨支撑所需的机械性能。这些经选择性激光熔化处理的WE43支架有望满足理想骨替代品的所有功能要求。 经体内研究发现镁基材料在体内环境中会发生局部点状腐蚀且生物降解率快,并在生物降解过程中释放氢气等均不利于植入物在体内应用。为了优化其理化性能,调节镁基降解速率,有学者将镁基与可降解高分子材料结合应用。LI等[37]使用低温3D打印技术来制备用于骨骼修复的含镁可降解聚合物,将具有成骨活性和血管生成活性的镁均匀地掺入乙交酯-丙交酯共聚物/磷酸三钙可降解多孔支架中,这种含镁的可降解聚合物骨修复材料具有良好的生物相容性和生物活性,机械强度明显提高,使之与松质骨匹配,并显著改善了植入物的再生和血管生成。 2.2.4 铁基金属材料 铁(Fe)是人体必需的微量元素之一,并参与各种生理反应,目前铁因其优异的机械性能和可降解性而被认为是制造可降解植入物的首选[38]。初步的体内实验结果表明,植入猪主动脉的纯铁支架不会引起局部或全身毒性反应[39],这是因为降解后释放的铁离子可以被人体吸收代谢而不会积累。但是纯铁在体内的降解率慢[40],且铁磁性会影响一些成像检测系统(MRI等)的检测,从而一定程度上限制了铁基材料在临床上的应用[41],因此增材制造旨在实现铁基材料更快的生物降解速度,改变铁磁特性并增强其生物相容性。CHOU等[42]在纯铁的基础上添加一定含量的锰(Mn)元素,利用3D打印制备Fe-30Mn可生物降解支架,经过烧结形成具有马氏体ε和奥氏体γ相的混合相合金,结果显示它的腐蚀速率比纯铁快,且拉伸机械性能与天然骨骼相似,从而减少折裂风险;除此之外,Fe-30Mn支架还显示出优异的细胞相容性。即使在体外将纯铁合金化可以加速其生物降解,但体内尚未观察到这种改善,还需要一定的临床试验以佐证这一结论。LI等[43]首先报道了使用直接金属印刷制备拓扑有序的多孔铁支架,并研究了直接金属印刷工艺对支架表面积和晶粒尺寸的影响,结果表明,即使降解4周多孔支架的力学性能仍在小梁骨的容许范围内,降解速率比冷轧铁快12倍,质量损失仅为3.1%。 除了铁基合金以外,还有许多针对铁和氧化铁颗粒的研究。ZHANG等[44]使用3D-Bioplotter打印机制备包含磁性四氧化三铁纳米颗粒和生物活性玻璃/聚己内酯的复合支架,使支架的孔隙率均匀分布在60%,直径为400 μm,抗压强度为13-16 MPa,并将阿霉素类抗癌药装载到该支架中以促进成骨细胞成骨,同时实现持续的药物递送。实验结果显示,由铁基合金制成的3D打印可生物降解多孔支架能够促进骨再生而无任何并发症,有望成为骨科植入物的良好候选者。 2.2.5 锌基金属材料 锌是人体中必不可少的微量元素之一,它直接参与体内的酶合成、核酸代谢、基因表达、信号转导、细胞凋亡调节、生长促进和组织再生,具有抗菌活性[45]。近年来,因为锌的降解率介于铁和镁之间,已逐渐取代铁和镁合金,使锌及其合金有望成为可降解心血管支架和牙科植入物的理想材料[46]。尽管通过3D打印技术可以制备出具有患者特定要求的可生物降解锌植入物,但锌的低熔点、低沸点和易于氧化的特性通常会导致制造零件高孔隙率,影响其机械性能。DEMIR等[47]首先研究了使用选择性激光融化制造可降解生物的纯锌植入物,并提出了制造过程的参数,结果显示3D打印的纯锌零件显微硬度比传统制备的略高,通过调整选择性激光融化锌部件的密度、微观结构和几何形状可改变产品的机械性能和降解。其后,WEN等[48]通过选择性激光融化制备出直径为2-5 mm、支杆直径为200-500 μm的纯锌心血管支架,证明了选择性激光融化技术具有制备复杂结构的能力。目前的研究结果表明,选择性激光融化将取代传统的固体纯锌零件加工方法和按地形顺序排列的纯锌心血管支架,这在生物医学领域具有令人鼓舞的前景。 2.2.6 钽基金属材料 钽具有“亲生物”金属之称,其独特特性包括低弹性模量、耐腐蚀性、优异骨整合特性、生物相容性、组织向内生长特性及高表面摩擦力。临床研究已经验证多孔钽植入物在各种临床环境中的应用,并尝试使用新的制造技术来制造多孔或固态钽零件。BALLA等[49]首次通过激光工程网成形在钛上涂覆钽,与钛表面相比,钽涂层显著改善了骨整合性能。随后,他们还使用激光工程网成形制备了具有不同孔隙率的钽多孔结构[50],该制造方法通过将孔隙率调整在27%-55%之间使其杨氏模量范围为1.5-20 GPa,而且多孔钽结构促进细胞的黏附、增殖、分化,甚至早期生物固定。但由于钽的熔点超过3 000 ℃,使得市场上的大多数3D打印设备无法使用钽粉。为解决这一问题,有学者提出应用可以完全溶解钽粉的选择性激光熔化技术生产孔隙率几乎为零的高强度零件。WAUTHLE等[51]使用选择性激光融化制备具有相互连接的多孔纯钽植入物,与多孔TC4相比具有更加优异的骨传导性能、更高的抗疲劳强度和高延展性,证实了通过选择性激光融化技术可生产具有可调机械性能和生物性能的多孔钽植入物。 2.2.7 其他金属基材料 在生物医学领域中,钛、钴铬合金等金属是研究及应用较为成熟的金属基生物材料,316L不锈钢、铜(Cu)也因其优良的理化特性受到广泛关注。316L不锈钢是生物医学应用中常用的金属之一[52],虽然316L不锈钢中碳含量相对较低、耐腐蚀性良好,但通过观察其在高应力耗氧环境下发生腐蚀的可能性明显增大[53-54],尽管如此,316L不锈钢仍因价格低廉及机械性能和生物相容性优越等优势成为前景可观的生物相容性材料[55]。因此目前研究人员对316L不锈钢的研究主要集中在应用选择性激光融化的工艺参数来改善所制造零件的几何和微观结构特性,如表面粗糙度、表面完整性、高密度和残余应力[56-58]。铜在选择性激光融化过程中预热温度较低,粉末流动性好,成形件的后处理较为容易,但对激光反射率较高,从而造成未熔、孔洞、裂纹等缺陷,降低了加工零件的质量和性能。目前铜打印的研究热点与难点在于如何提高铜对激光的吸收,以优化增材制作工艺[59]。 2.3 3D打印金属基生物材料临床应用 金属3D打印技术在生物医学领域中的兴起极大地引领了其在整形外科和牙科植入物中的应用,以下将对金属基生物材料在骨科、口腔科及心血管设备的临床应用加以阐述。 2.3.1 骨科应用 骨科领域是金属3D打印技术发展之后最具有发展潜力的生物医学领域之一[60]。由于人体的多样性和骨缺损部位的随机性,金属3D打印技术模仿自然结构(骨骼等)特性的复杂设计能力是其他传统制造方法所无法比拟的,同时能够实现对患者进行“量体裁衣”的治疗方案,满足不同人群对精准医疗的盼望与需求[61]。郭征教授带领的团队采用金属 3D 打印技术制备与患者锁骨和肩胛骨完全一致的钛合金植入假体,并通过手术成功将钛合金假体植入骨肿瘤患者体内,在世界范围内首次实现肩胛带不定形骨重建,标志着 3D 生物打印个体化金属骨骼修复技术的进一步成熟[62]。金属基骨修复支架是骨科植入物替代人体器官的最佳选择之一,3D打印为制造具有复杂设计、高度互联孔隙的支架开辟了新的可能性[63]。已有学者通过实验证明,选择性激光融化和电子束熔化具有尺寸精度高、成型分辨率高、环境清洁、材料节约、可定制性高等优点,在金属细胞支架制造中具有巨大的潜力[64]。 2.3.2 口腔医学应用 口腔金属生物材料常用于修复牙体缺损、牙列缺失、牙齿矫形及颌面部骨重建。由于口腔内呈弱碱性环境且需承受一定的咀嚼力,这种特殊的微环境使口腔的金属材料发生易腐蚀变化,金属离子及其衍生物析出,长期与机体直接接触后机体将发生过敏、细胞毒性等不良反应[65]。因此,口腔金属材料应具有良好生物相容性、耐腐蚀性能、耐磨性,目前较为常用的钴铬合金和钛合金[66]。 在口腔修复科的应用中,金属生物材料主要用于金属冠桥、活动义齿支架等的制作,其中由选择性激光融化技术所制作的钴铬合金固定冠桥应用最为广泛[67]。实验表明,由3D打印技术制备的金属冠金瓷结合力和耐腐蚀性均明显优于铸造钴铬合金烤瓷金属基底冠[68-69]。HU等[70]利用口内扫描和选择性激光融化技术制作以钛基为支架的活动义齿,替代了传统压印和浇铸技术,减少了椅旁时间,增加了义齿使用舒适度,质量稳定,证明利用3D打印技术制作金属材料修复体能有效提高修复体多项性能,操作简便,适合应用于临床。 传统正畸固定矫治器多位于唇侧,美观性差,而舌侧矫治器虽增加美观性,但易引起舌部不适、影响发音和进食。林泽等[71]利用3D打印设计个性化舌侧托槽,使之更加贴合患者牙面,符合患者个人特征,吻合度更高,减少了托槽脱落和不适等并发症的发生,同时简化了临床操作,证实3D打印舌侧托槽其力学性能(抗拉强度,表面硬度)优于铸造托槽。 自从BR?NEMARK首次发现钛与骨组织之间的骨结合现象以来,由TC4制成的骨质螺钉状牙科植入物如今已大量商业化生产并临床用于牙科[72]。种植牙要求具有良好的骨结合能力、负重能力、抗疲劳能力和材料强度;多孔结构钛(在支架结构中)的宏观结构修饰可以改变其在生物相容性行为上的功能输出,且进一步扩大特定的接触面积,减少应力屏蔽,从而影响骨与植入物界面处的骨形成。制造商已经使用3D打印技术制造出了具有多孔或粗糙表面的新型牙科植入物[73]。近年来即刻种植被越来越多专家学者关注,MANGANO等[74]根据需拔除牙根的形态设计由选择性激光融化技术制备TC4根形种植体,在牙根拔除后将种植体即刻种植于拔牙窝,种植体和拔牙窝早期发生紧密结合,有利于后期获得良好的稳定性,1年成功率为100%,证实选择性激光融化技术制造的个性化种植体可成功地应用于临床实践。 由于颌面部解剖结构复杂及美观性要求高,使得对于颌面部先天性或后天性缺陷的重建极具挑战性。自体骨移植曾被认为是颅面骨骼重建的金标准[75],然而它们的使用受到许多因素的限制。目前随着3D打印技术的进步及应用,极大程度上促进了更精确和可预测的颌面部重建手术。LEISER等[76]利用选择性激光烧结制备钛合金下颌假体,并将假体连接到咀嚼肌肉,为下颌骨粉碎性骨折患者实现下颌骨的重建,达到良好的功能和美学恢复效果,术后6个月随访骨移植物融合良好,开口度恢复至40 mm,同时也证实了3D打印技术可以成为制备颌骨重建个性化植入物的理想方法。但3D打印金属材料是否适合长期应用于人体还需要未来大规模的临床试验。 2.3.3 血管外科 随着微创技术的应用及发展,使各种心血管疾病的治疗转向介入治疗。目前,血管支架多为传统的标准生产,与人体血管的直径和形态存在个体差异,难以满足个性化需求[77];3D打印不仅可以在短时间内实现个性化的血管支架,还可以避免传统支架依从性差而导致的治疗失败。SUN等[78]利用血管造影重建血管三维解剖结构,并在重建模型上通过3D打印制作适合患者的个性化血管支架,证实了在3D打印冠状动脉模型中模拟冠状动脉支架的可行性。王江平等[79]成功制备了个性化3D打印钛合金多孔静脉血管外支架,并完成了世界上第一个利用增材制造完成胡桃夹综合征血管外支架植入,术后患者恢复良好;随后,前瞻性收集2015年8月至2016年8月唐都医院10例通过3D打印血管外支架治疗胡桃夹综合征患者的资料,术后随访患者均无症状,且支架稳定未发生移位,证实3D打印血管外支架植入是可以成为一种安全、有效且微创治疗胡桃夹综合征的方法[79]。虽然目前的3D打印血管支架集中在可生物吸收聚合物支架上,但这种高分子支架材料仍无法达到金属支架支撑强度高、抗压强度等治疗要求[80];随着3D打印金属材料不断研究,3D打印金属血管支架一定能够在腔内血管治疗领域占有一席之地。 "
| [1] CHEN Q, THOUAS GA. Metallic implant biomaterials. Mater Sci Eng C. 2015;87:1-57. [2] 张永涛,刘汉源,王昌,等.生物医用金属材料的研究应用现状及发展趋势[J].热加工工艺, 2017,46(4):21-26. [3] PRASAD K, BAZAKA O, CHUA M, et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials. 2017;10(8):884. [4] NI J, LING H, ZHANG S, et al. Three-dimensional printing of metals for biomedical applications. Mater Today Bio. 2019;3:100024. [5] SUSKA F, KJELLER G, TARNOW P, et al. Electron Beam Melting Manufacturing Technology for Individually Manufactured Jaw Prosthesis: A Case Report. Oral Maxillofac Surg. 2016;74(8):1706.e1-1706.e15. [6] MURR LE, MARTINEZ E, AMATO KN, et al. Fabrication of Metal and Alloy Components by Additive Manufacturing: Examples of 3D Materials Science. J Mater Res Technol. 2012;1(1):42-54. [7] JIANG R, MOSTAFAEI A, WU Z, et al. Effect of heat treatment on microstructural evolution and hardness homogeneity in laser powder bed fusion of alloy 718. Addit Manuf. 2020;35:101282. [8] PATTANAYAK DK, FUKUDA A, MATSUSHITA T, et al. Bioactive Ti metal analogous to human cancellous bone: Fabrication by selective laser melting and chemical treatments. Acta Biomater. 2011;7(3):1398-1406. [9] SAMES WJ, LIST FA, PANNALA S, et al. The metallurgy and processing science of metal additive manufacturing. Int Mater Rev. 2016;61(5):315-360. [10] GOKULDOSS PK, KOLLA S, ECKERT J. Additive Manufacturing Processes: Selective Laser Melting, Electron Beam Melting and Binder Jetting-Selection Guidelines. Materials. 2017;10(6):672. [11] TYRALLA D, SEEFELD T. Advanced Process Monitoring in Additive Manufacturing. PhotonicsViews. 2020;17(3):60-63. [12] HARUN WSW, KAMARIAH MSIN, MUHAMAD N, et al. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018;327:128-150. [13] KUMAR A, MANDAL S, BARUI S, et al. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater Sci Eng R. 2016;103:1-39. [14] SPENCER OO, YUSUF OT, TOFADE TC. Additive Manufacturing Technology Development: A Trajectory Towards Industrial Revolution. Mech Ind Eng. 2018;3(5):80-90. [15] PODSHIVALOV L, GOMES CM, ZOCCA A, et al. Design, Analysis and Additive Manufacturing of Porous Structures for Biocompatible Micro-Scale Scaffolds. Procedia CIRP. 2013;5:247-252. [16] HAMIDI MFFA, HARUN WSW, SAMYKANO M, et al. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater Sci Eng C. 2017;78:1263-1276. [17] SHAH FA, SNIS A, MATIC A, et al. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016;30: 357-367. [18] ZHANG LC, ATTAR H, CALIN M, et al. Review on manufacture by selective laser melting and properties of titanium based materials for biomedical applications. Mater Technol. 2016;31(2):66-76. [19] AZIZ IA, GABBITAS B, STANFORD M. Direct Metal Laser Sintering of a Ti6Al4V Mandible Implant. Key Eng Mater. 2012;520:220-225. [20] ASSIOTIS A, TO K, MORGAN-JONES R, et al. Patellar complications following total knee arthroplasty: a review of the current literature. Eur J Orthop Surg Traumatol. 2019;29(8):1605-1615. [21] GAO CH, WANG CY, JIN H, et al. Additive manufacturing technique-designed metallic porous implants for clinical application in orthopedics. RSC Adv. 2018;44(8):25210-25227. [22] TAN XP, TAN YJ, CHOW CSL, et al. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater Sci Eng C. 2017;76:1328-1343. [23] SIDAMBE AT. Biocompatibility of Advanced Manufactured Titanium Implants-A Review. Materials. 2014;7(12):8168-8188. [24] MACBARB RF, LINDSEY DP, WOODS SA, et al. Fortifying the Bone-Implant Interface Part 2: An In Vivo Evaluation of 3D-Printed and TPS-Coated Triangular Implants. Int J Spine Surg. 2017;11(3):16. [25] WYSOCKI B, IDASZEK J, ZDUNEK J, et al. The Influence of Selective Laser Melting (SLM) Process Parameters on In-Vitro Cell Response. Int J Mol Sci. 2018;19(6):1619. [26] PALMQUIST A, SNIS A, EMANUELSSON L, et al. Long-term biocompatibility and osseointegration of electron beam melted, free-form-fabricated solid and porous titanium alloy: experimental studies in sheep. J Biomater Appl. 2013;27(8):1003-1016. [27] ZANGENEH S, LASHGARI HR, ROSHANI A. Microstructure and tribological characteristics of aged Co-28Cr-5Mo-0.3C alloy. Mater Des. 2012;37(5): 292-303. [28] KOUTSOUKIS T, ZINELIS S, ELIADES G, et al. Selective Laser Melting Technique of Co-Cr Dental Alloys: A Review of Structure and Properties and Comparative Analysis with Other Available Techniques. J Prosthodont. 2015;24(4):303-312. [29] LU Y, WU S, GAN Y, et al. Investigation on the microstructure, mechanical property and corrosion behavior of the selective laser melted CoCrW alloy for dental application. Mater Sci Eng C. 2015;49:517-525. [30] HAZLEHURST K, WANG CJ, STANFORD M. Evaluation of the stiffness characteristics of square pore CoCrMo cellular structures manufactured using laser melting technology for potential orthopaedic applications. Mater Des. 2013;51:949-955. [31] WANG L, KANG J, SUN C, et al. Mapping porous microstructures to yield desired mechanical properties for application in 3D printed bone scaffolds and orthopaedic implants. Mater Des. 2017;133:62-68. [32] SAHASRABUDHE H, BOSE S, BANDYOPADHYAY A. Laser processed calcium phosphate reinforced CoCrMo for load-bearing applications: Processing and wear induced damage evaluation. Acta Biomater. 2018;66:118-128. [33] HAN P, CHENG P, ZHANG S, et al. In vitro and in vivo studies on the degradation of high-purity Mg (99.99wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials. 2015;64:57-69. [34] HAUDE M, INCE H, ABIZAID A, et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016;387(10013):31-39. [35] JAUER L, JÜLICH B, VOSHAGE M, et al. Selective Laser Melting of magnesium alloys. Eur Cells Mater. 2015;30:1. [36] LI Y, ZHOU J, PAVANRAM P, et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018;67:378-392. [37] LI C, LAI Y, LI L, et al. The in vitro biocompatibility and osteoinductive activity study of magnesium composed PLGA/TCP porous scaffold for bone regeneration. J Orthop Translat. 2016;7:78. [38] DO AV, KHORSAND B, GEARY SM, et al. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv Healthc Mater. 2015;4(12): 1742-1762. [39] KRAUS T, MOSZNER F, FISCHERAUER S, et al. Biodegradable Fe-based alloys for use in osteosynthesis: outcome of an in vivo study after 52 weeks. Acta Biomater. 2014;10(7):3346-3353. [40] LIU B, ZHENG YF. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011;7(3):1407-1420. [41] 郑玉峰,吴远浩.处在变革中的医用金属材料[J].金属学报,2017,53(3): 257-297. [42] CHOU DT, WELLS D, HONG D, et al. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013;9(10): 8593-8603. [43] LI Y, JAHR H, LIETAERT K, et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018;77:380-393. [44] ZHANG J, ZHAO S, ZHU M, et al. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J Mater Chem B. 2014; 2(43):7583-7595. [45] MOSTAED E, SIKORA-JASINSKA M, MOSTAED A, et al. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J Mech Behav Biomed Mater. 2016;60:581-602. [46] MOSTAED E, SIKORA-JASINSKA M, DRELICH JW, et al. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018;71:1-23. [47] DEMIR AG, MONGUZZI L, PREVITALI B. Selective laser melting of pure Zn with high density for biodegradable implant manufacturing. Addit Manuf. 2017;15:20-28. [48] WEN P, VOSHAGE M, JAUER L, et al. Laser additive manufacturing of Zn metal parts for biodegradable applications: Processing, formation quality and mechanical properties. Mater Des. 2018;155:36-45. [49] BALLA VK, BANERJEE S, BOSE S, et al. Direct laser processing of a tantalum coating on titanium for bone replacement structures. Acta Biomater. 2010; 6(6):2329-2334. [50] BALLA VK, BODHAK S, BOSE S, et al. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010;6(8):3349-3359. [51] WAUTHLE R, VAN DER STOK J, AMIN YAVARI S, et al. Additively manufactured porous tantalum implants. Acta Biomater. 2015;14:217-225. [52] SING SL, AN J, YEONG WY, et al. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J Orthop Res. 2016;34(3):369-385. [53] AHERWAR A, SINGH AK, PATNAIK A. Current and future biocompatibility aspects of biomaterials for hip prosthesis. AIMS Bioeng. 2016;3(1):23-43. [54] ASRI RIM, HARUN WSW, SAMYKANO M, et al. Corrosion and surface modification on biocompatible metals: A review. Mater Sci Eng C. 2017;77: 1261-1274. [55] KAMARIAH MSIN, HARUN WSW, KHALIL NZ, et al. Effect of heat treatment on mechanical properties and microstructure of selective laser melting 316L stainless steel. IOP Conference Series: Mater Sci Eng. 2017;257(1): 012021. [56] SIMSON T, EMMEL A, DWARS A, et al. Residual stress measurements on AISI 316L samples manufactured by selective laser melting. Addit Manuf. 2017;17:183-189. [57] DEEV AA, KUZNETCOV PA, PETROV SN. Anisotropy of Mechanical Properties and its Correlation with the Structure of the Stainless Steel 316L Produced by the SLM Method. Phys Procedia. 2016;83:789-796. [58] LI X, WILLY HJ, CHANG S, et al. Selective laser melting of stainless steel and alumina composite: Experimental and simulation studies on processing parameters, microstructure and mechanical properties. Mater Des. 2018; 145:1-10. [59] 杨睿,黎振华,李淮阳,等.选区熔化3D打印铜的研究进展[J].稀有金属,2020,8(1):1-11. [60] CAI H. Application of 3D printing in orthopedics: status quo and opportunities in China. Ann Transl Med. 2015;3(Suppl 1):S12. [61] 罗文峰,杨雪香,敖宁建,等.生物医用材料的3D打印技术与发展[J].材料导报,2016,30(13):81-86. [62] 丁香园.世界首例3D打印金属锁骨和肩胛骨植入手术在西京医院成功实施[J].生物骨科材料与临床研究,2014,11(3):34. [63] BOSE S, VAHABZADEH S, BANDYOPADHYAY A. Bone tissue engineering using 3D printing. Mater Today. 2013;16(12):496-504. [64] WANG Z, WANG C, LI C, et al. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: A review. J Alloys Compd. 2017;717:271-285. [65] 于华,张晓东,王亦菁,等.口腔金属材料安全性、相容性以及功能性的评价[J].中国组织工程研究,2012,16(47):8907-8914. [66] SALOU L, HOORNAERT A, LOUARN G, et al. Enhanced osseointegration of titanium implants with nanostructured surfaces: an experimental study in rabbits. Acta Biomater. 2015;11:494-502. [67] 李国强,沈晴昳,高建华,等.选择性激光熔覆钴铬合金与瓷的结合性能研究[J].实用口腔医学杂志,2012,28(3):302-305. [68] 张格.钴铬合金义齿选区激光熔化及热处理工艺研究[D].太原:中北大学,2017. [69] 忻贤贞,项楠,陈洁,等.选择性激光熔化技术制作牙科钴铬合金的电化学腐蚀性能研究[J].上海交通大学学报(医学版),2012,32(5):76-78. [70] HU F, PEI Z, WEN Y. Using Intraoral Scanning Technology for Three-Dimensional Printing of Kennedy Class I Removable Partial Denture Metal Framework: A Clinical Report. J Prosthodont. 2019;28(2):e473-e476. [71] 林泽,陈军,李雪. 数字化3D打印技术在口腔舌侧正畸托槽粘接中应用研究[J].中国实用口腔科杂志,2016,9(2):104-107. [72] REVATHI A, BORRÁS AD, MUÑOZ AI, et al. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater Sci Eng C. 2017;76:1354-1368. [73] ZENA W, VAN GRUNSVEN W, CLAEYSSENS F, et al. Porous Titanium for Dental Implant Applications. Metals. 2015;5(4):1902-1920. [74] MANGANO FG, CIROTTI B, SAMMONS RL, et al. Custom-made, root-analogue direct laser metal forming implant: a case report. Lasers Med Sci. 2012;27(6):1241-1245. [75] SAKKAS A, WILDE F, HEUFELDER M, et al. Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent. 2017;3(1):23. [76] LEISER Y, SHILO D, WOLFF A, et al. Functional Reconstruction in Mandibular Avulsion Injuries. J Craniofac Surg. 2016;27(8):2113-2116. [77] 刘博文,陈忠,王盛,等.3D打印技术在血管外科领域应用现状及展望[J].中国实用外科杂志,2016,36(3):341-343. [78] SUN Z, JANSEN S. Personalized 3D printed coronary models in coronary stenting. Quant Imaging Med Surg. 2019;9(8):1356-1367. [79] 王江平,焦勇,许志斌,等.腹腔镜3D打印血管外支架植入术治疗胡桃夹综合征的安全性和有效性[J]. 中华泌尿外科杂志,2018,39(3):200-204. [80] YANG L, CHEN X, ZHANG L, et al. Additive Manufacturing in Vascular Stent Fabrication. Proceedings of 2018 International Conference on MSME. 2019;253(11):03003. [81] XIE FX, HE XB, CAO SL, et al. Influence of pore characteristics on microstructure, mechanical properties and corrosion resistance of selective laser sintered porous Ti–Mo alloys for biomedical applications. Electrochimica Acta. 2013;105:121-129. [82] XIE F, HE X, LU X, et al. Preparation and properties of porous Ti-10Mo alloy by selective laser sintering. Mater Sci Eng C. 2013;33(3):1085-1090. [83] STRUZIKIEWICZ G, ZĘBALA W, MATRAS A, et al. Turning Research of Additive Laser Molten Stainless Steel 316L Obtained by 3D Printing. Materials (Basel). 2019;12(1):182. [84] GAO W, ZHANG Y, RAMANUJAN D, et al. The status, challenges, and future of additive manufacturing in engineering. Comput Aided Design. 2015;69: 65-89. [85] Guo N, Leu MC. Additive manufacturing: technology, applications and research needs. Front Mech Eng. 2013;8(3):215-243. [86] ESPANA FA, BALLA VK, BOSE S, et al. Design and fabrication of CoCrMo alloy based novel structures for load bearing implants using laser engineered net shaping. Mater Sci Eng C. 2010;30(1): 50-57. [87] MALEK NMSA, MOHAMED SR, GHANI SAC, et al. Critical Evaluation on Structural Stiffness of Porous Cellular Structure of Cobalt Chromium Alloy. Iop Conference. 2015;100(1):1-10. [88] MARATTUKALAM JJ, SINGH AK, DATTA S, et al. Microstructure and corrosion behavior of laser processed NiTi alloy. Mater Sci Eng C. 2015;57:309-313. [89] ZHAI Y, GALARRAGA H, LADOS DA. Microstructure Evolution, Tensile Properties, and Fatigue Damage Mechanisms in Ti-6Al-4V Alloys Fabricated by Two Additive Manufacturing Techniques. Procedia Eng. 2015;114:658-666. [90] YU J, ROMBOUTS M, MAES G, et al. Material Properties of Ti6Al4V Parts Produced by Laser Metal Deposition. Phys Procedia. 2012;39:416-424. [91] BALLA VK, BHAT A, BOSE S, et al. Laser processed TiN reinforced Ti6Al4V composite coatings. J Mech Behav Biomed Mater. 2012;6:9-20. [92] SINGH S, RAMAKRISHNA S. Biomedical applications of additive manufacturing: Present and future. Curr Opin Biomed Eng. 2017;2: 105-115. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [3] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [4] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [5] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [6] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [7] | Jiang Hongying, Zhu Liang, Yu Xi, Huang Jing, Xiang Xiaona, Lan Zhengyan, He Hongchen. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1149-1153. |
| [8] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [9] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [10] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [11] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [12] | Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling. Photoreceptor cell replacement therapy for retinal degeneration diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1116-1121. |
| [13] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| [14] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [15] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||