Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (10): 1616-1621.doi: 10.3969/j.issn.2095-4344.3064
Previous Articles Next Articles
Contribution of electrofabrication technology to the progress of tissue engineering research
Lü Pengcheng1, 2, 3, Zhang Qian1, 2, Mao Jifu1, 2, 3, Lao Jihong1, 2, Wang Lu1, 2, 3
- 1College of Textile, Donghua University, Shanghai 201620, China; 2Key Laboratory of Textile Science & Technology, Ministry of Education, Donghua University, Shanghai 201620, China; 3Key Laboratory of Biomedical Textile Materials and Technology in Textile Industry, Donghua University, Shanghai 201620, China
-
Received:2020-04-23Revised:2020-04-26Accepted:2020-06-17Online:2021-04-08Published:2020-12-18 -
Contact:Mao Jifu, Researcher, Doctoral supervisor, College of Textile, Donghua University, Shanghai 201620, China; Key Laboratory of Textile Science & Technology, Ministry of Education, Donghua University, Shanghai 201620, China; Key Laboratory of Biomedical Textile Materials and Technology in Textile Industry, Donghua University, Shanghai 201620, China -
About author:Lü Pengcheng, Master candidate, College of Textile, Donghua University, Shanghai 201620, China; Key Laboratory of Textile Science & Technology, Ministry of Education, Donghua University, Shanghai 201620, China; Key Laboratory of Biomedical Textile Materials and Technology in Textile Industry, Donghua University, Shanghai 201620, China -
Supported by:the Fundamental Research Funds for the Central Universities, No. 2232020G-01 (to MJF); the Research Start-up Funds for High-Level Talents of Donghua University (to MJF); the Subject Innovation and Talent Introduction Program in Colleges and Universities, No. BP0719035 (to WL)
CLC Number:
Cite this article
Lü Pengcheng, Zhang Qian, Mao Jifu, Lao Jihong, Wang Lu. Contribution of electrofabrication technology to the progress of tissue engineering research[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1616-1621.
share this article
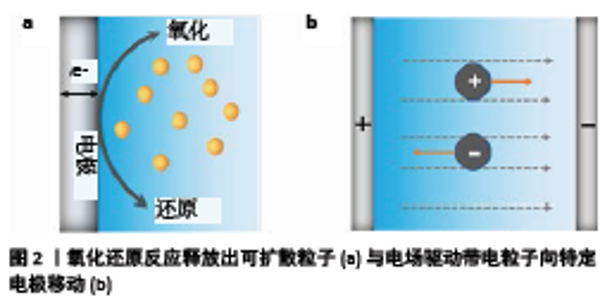
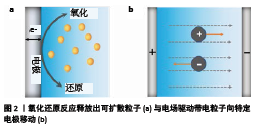
2.1 生物材料电加工机制 许多生物衍生的聚合物都具有刺激响应的自组装特性,可以通过化学、物理信号的刺激形成具有一定结构的组织工程支架[22]。一些刺激响应的自组装生物衍生聚合物可以通过电信号(电流或电场)触发而沉积在电极表面上。根据目前的研究,可以将电加工机制分为2种:电化学反应和电场驱动作用。 2.1.1 电化学反应 电化学反应涉及电子在电极表面上的转移,阳极和阴极分别发生氧化反应和还原反应。电解液中的粒子可以直接在电极表面发生氧化还原反应从而沉积在电极表面[11-12,23],这种情况的典型例子就是金属电镀工艺。在另一些情况下,电沉积过程基于电极上化学物质(可扩散粒子)的生成,如图2a所示,然后向溶液扩散,这些可扩散粒子与电解液中的大分子直接反应或者引起局部pH值变化,从而引发电沉积[17-18,24-25]。根据CROSS[26]的研究,这些可扩散粒子能够在距电极表面微纳米尺度内引导分子组装。 由pH值梯度引发电沉积聚合物的典型例子就是壳聚糖的阴极电沉积[27-28]。阴极电解水反应可导致阴极附近pH值升高(> 6.3),使壳聚糖链局部去质子化,进而使其凝胶化。相反,阳极电解反应会在阳极附近产生一个低pH值的区域,YOKOYAMA等[29]报道了海藻酸盐在阳极的低pH值区域形成块状凝胶这一现象。随后CHEONG等[30]开发了一种由Ca2+引发的藻酸盐成膜方法,在电解液中加入碳酸钙微粒,在阳极的低pH值区域碳酸钙溶解释放出Ca2+,从而引发交联形成凝胶。此外,Fe3+也可引发海藻酸盐凝胶的生成。BRUCHET 等[31]通过电化学反应改变电解液中铁离子的价态制备了藻酸盐可逆凝胶。在一些情况下,通过氧化还原反应产生的一些产物也可以引发交联。GENG等[32]使用铜电极为阳极使其电化学氧化,生成的Cu2+可引发壳聚糖薄膜的生成,这一过程是不可逆的。 2.1.2 电场驱动作用 一些具有极性的生物高分子或者带电粒子,在电场力的作用下会定向排列并受驱动向着某一电极迁移,如图2b所示[33]。GARGAVA等[17]据此提出了一种方法,将阳极插入负载有Ca2+的琼脂糖模板中,在静电力作用下Ca2+从模板中向外释放出来,而海藻酸根则会向阳极迁移,以此引导海藻酸盐在模板上形成凝胶。此外,还可以利用电场力在组织工程支架上负载生物活性物质[14],或者在材料表面覆盖功能涂层[34-35]。除此之外,电场对沉积材料的结构有一定影响。YAN等[33]的研究表明通过电场的“开/关”,沉积的壳聚糖水凝胶出现明显的分层效应,通过电场的调控可以创建分层结构。外加的电场还可以使一些生物高分子极化并按一定方向排列对齐,赋予材料结构各向异性[36]。与电化学反应相比,人们对电场的关注较少,需要更多的研究来探究其影响规律。 "
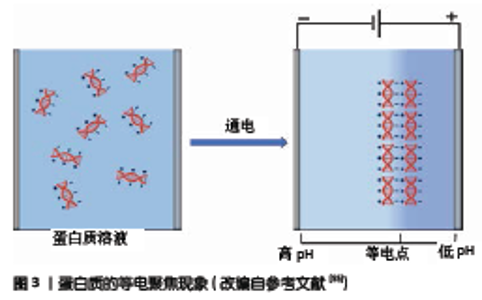
2.2 结构调控 通过电加工方法制备的生物材料有许多优点,比如在很短的时间内(通常在几分钟内)就能制得所需的材料,并且可以通过改变电信号的输入精准调控材料结构;加工过程通常只需要沉积液,加工环境温和,因此也适用于生物大分子(如蛋白质、多糖、核酸等)和细胞等的封装。该部分主要讨论电加工过程的结构调控。在电加工的研究中通常会提到“时空控制”这一术语,时空控制是用于通过控制时间和空间参数(控制生长速率以及所得材料的形状和厚度等)来描述材料加工的术语[33,37]。 刺激响应型生物高分子材料可以在具有任何几何形状的电极表面制备,可以是具有3D空间结构的电极或者图案化的平面电极,也可以用移动电极笔进行电书写[38-39]。在固定电极体系中,可以通过对电极表面的刻蚀或者涂覆使电极上存在导电/非导电区域,从而仅在电极的导电区域形成图案化材料[13,40]。除了在二维平面中形成凝胶外,还可以在具有三维结构的电极上制备具有立体形状的凝胶。BRESSNER等[41]使用弯曲的环状电极成功制备了具有三维空间结构的丝素薄膜,在移动电极体系中电极笔的直径、书写速度和保持时间可以控制所形成凝胶“笔迹”的厚度和宽度,如果将电极笔与计算机控制的机械臂连接,可以达到更精确的结构控制[38]。例如在含有壳聚糖的琼脂模板表面以阴极为“笔”进行电书写,利用局部高pH值引发壳聚糖凝胶形成“笔迹”。 通过对输入电信号的控制也能调控材料结构。有研究表明,沉积的膜或水凝胶厚度与电流输入时间(转移的电荷)呈线性正相关[15]。通过电信号调控易于构建具有层状结构的凝胶,可以通过周期电信号输入在玻璃碳电极上生成多层分段的壳聚糖水凝胶,每个周期所生成的水凝胶之间具有明显边界,控制电信号还允许凝胶的顺序组装,制得分层多组分胶凝系统[42]。控制施加的电流还可以选择性地触发多组分系统中具有不同pKa值的胶凝剂,使其根据pH值变化依次形成凝胶[40]。此外,电解液中盐的浓度对水凝胶的结构也有重要影响,无机盐离子对电场具有屏蔽作用,能够影响壳聚糖的自组装,从而减弱材料微观结构的取向性[42]。在一定范围内盐溶液浓度的增大可使壳聚糖沉积的速率增大,并且使膜表面变得更粗糙[43]。在盐存在的情况下可能导致形成分子间氢键的可用位点减少,使壳聚糖链更易塌陷且具有柔性,从而降低材料机械强度,并更易在材料内部形成孔隙结构[37,44]。 2.3 适用于电加工的生物高分子材料 生物材料电加工是一个相对较新的研究领域,目前报道的基于电加工的生物活性材料主要集中于壳聚糖、海藻酸、胶原蛋白和丝素蛋白等材料上。 2.3.1 壳聚糖 壳聚糖是一种阳离子多糖,作为生物医用材料在药物缓释、细胞培养载体、组织工程材料方面具有巨大的潜在应用价值。2002年,WU等[28]使用天然多糖壳聚糖溶液首次实现了壳聚糖的阴极电沉积,在电沉积聚合物领域开辟了新的一页。由于伯氨基的质子化,壳聚糖在pH值< pKa(约6.3)值下可溶,在pH值>pKa值下不溶。壳聚糖的阴极沉积过程可以分为2步[45]:带电的壳聚糖链在电场作用下向电极的电泳运动;由于电极附近的pH值变化引起壳聚糖沉淀。CHENG等[46]使用光学显微镜下的微流体装置证实在电极附近存在pH值梯度,从而触发了半结晶壳聚糖凝胶的形成。 有关壳聚糖的阴极电沉积,人们已经对其进行了较多研究,证明其具有操作简单、加工条件温和无试剂及精确的时空控制等优势[37,47],电极图案化加工和电书写也都适用于壳聚糖。除此之外,还可以将其他材料(纳米颗粒、微胶囊、生物大分子等)与壳聚糖基质共沉积以增强凝胶功能性,并且电沉积可以保形地涂覆体内植入物复杂的表面[16]。在电沉积过程中,阴极上的电化学反应会产生H2气泡,被材料截留后具有致孔作用。然而也有研究者指出,这些H2气泡会影响凝胶表面光滑度并可能使材料产生结构缺陷。为了改善这一问题,GITERU等[48]使用脉冲电流进行阴极电沉积,能有效控制H2的释放,从而改善材料结构均匀性。另外在含有过氧化氢的电解液体系中,阴极不产生氢气,有利于材料结构的调控。壳聚糖还具有出色的配位过渡金属离子的能力,因此可以采用牺牲阳极的方法进行壳聚糖沉积。GENG等[32]使用铜作为阳极,利用电化学氧化反应原位产生Cu2+的配位作用来生成具有光滑均匀表面的壳聚糖薄膜。GRAY等[49]报道了另一种阳极电沉积的机制,在含有NaCl并具有缓冲能力的电解液体系中,NaCl的电解生成反应性氯物质,该物质会部分氧化壳聚糖并生成可与胺共价偶联的醛,从而生成共价键交联的壳聚糖薄膜。此外,在类似体系中还可以对壳聚糖材料进行改性。QU等[15]通过两步法制备了壳聚糖抗菌敷料,首先通过阴极电沉积法在电极表面制备壳聚糖膜,随后再将其作为阳极,在含有NaCl的磷酸盐缓冲液中对其进行氯化,生成含有N-Cl键的壳聚糖敷料,具有更强的抗菌作用。 2.3.2 海藻酸盐 海藻酸盐是天然的阴离子多糖杂聚物,是一种对多价态金属离子(例如Ca2+)响应的自组装多糖,其作为细胞/酶等的固定材料被广泛研究[50]。CaCl2作为交联剂在海藻酸盐凝胶的制备中被广泛应用,直接将其与海藻酸盐混合可快速引发交联反应,无法控制凝胶结构的均一和图案化。研究者期望通过Ca2+的可控释放来受控地加工藻酸盐水凝胶,因此开发了海藻酸盐凝胶的电化学制备方法。 最初有研究报道了H-Alg凝胶的电加工[29],使用海藻酸钠作为电解液,凝胶形成机制是由于水的电解产生H+影响海藻酸钠的解离平衡,从而在阳极附近生成了海藻酸凝胶,然而该过程仅能生成非连续块状凝胶。随后出现了一种基于Ca2+的电加工体系[30],向海藻酸钠溶液中添加不溶性CaCO3颗粒,通过阳极反应释放的H+与CaCO3反应进而原位释放Ca2+引发交联反应,从而可控地生成藻酸盐薄膜或者凝胶。GARGAVA等[17]在研究中指出,这种因pH值引发的Ca2+释放在电信号关闭后仍会存在一定时间,对精确控制凝胶结构不利,于是提出了该加工体系的改进方案,在阳极外包覆负载Ca2+的琼脂糖模板,在电场存在下由于电泳作用藻酸根会向阳极迁移,而Ca2+会从模板中释放出来,最终在模板表面形成海藻酸盐水凝胶。由于Ca2+的释放是受电场力驱动的,在停止通电后Ca2+的释放会立即停止,从而更精确地控制海藻酸盐凝胶的生成。此外,BRUCHET等[31]报道了通过铁离子的价态变化引发海藻酸盐凝胶的方法,Fe2+和Fe3+具有不同的配位性质,Fe2+倾向于结合包含氮和硫原子的中性配体,而Fe3+优先结合带负电荷的配体(例如羧酸根)中的氧原子,并且当Fe2+的浓度小于25 mmol/L时不会引发藻酸盐的交联。因此可以通过铁离子的氧化还原来控制藻酸盐凝胶的形成与分解[51],这为通过电信号控制药物的负载和释放提供了新思路。 总之,藻酸盐的电沉积具有与壳聚糖电沉积相似的优点:加工条件温和;可通过电信号调控结构[52];可图案化沉积和电书写[17,53];可以对具有复杂形状的材料进行保形涂层[31];易于负载其他生物活性成分[18,51]。 2.3.3 胶原蛋白与丝素蛋白 蛋白质是一种两性生物高分子,当悬浮于水性液体中时其具有pH值依赖的表面电荷,在溶液pH值小于蛋白质等电点时其带正电,而pH值大于等电点值时则带负电[54]。蛋白质材料的电化学制备原理与电聚焦(一种电泳技术,可将蛋白质根据其等电点的不同进行分离)类似,如图3所示,电解反应会形成pH值梯度,在外部电场影响下蛋白质分子会向等电点迁移并定向排列形成具有一定结构的材料[55]。 胶原蛋白作为细胞外基质的重要组成部分一直是组织工程支架、伤口愈合生物材料、药物输送和神经引导基质的重要基体材料。人体组织器官中的胶原蛋白通常具有取向分布的特性,以实现一定的生理功能和力学性能。传统方法如溶剂浇筑法制得的胶原蛋白凝胶材料,其内部胶原蛋白大分子的排列通常是无序的,导致材料无法形成致密结构,使其应用受限[56]。研究者期望找到一种方法使得胶原蛋白分子能够可控组装。电加工的方法可以通过电场引导胶原蛋白分子定向排列,从而赋予材料各向异性。CHENG等[57]在研究中观察到通过电沉积制备的胶原蛋白材料结构致密,排列整齐,其强度比随机交联取向的胶原蛋白膜大得多,更适合用于人工肌腱。也有研究者通过脉冲电流、采用非水电解液体系等方法减少电极上气体的释放来制备致密胶原蛋白材料。电加工形成的胶原蛋白材料还可以通过进一步处理提高性能。UQUILLAS 等[58]使用缓冲液对电加工制得的胶原蛋白凝胶进行处理来促进胶原蛋白网络中的原纤形成,从而提高机械强度。总而言之,对胶原蛋白电加工的研究大都聚焦于构建分子排列具有一定取向性的致密胶原蛋白材料,以便更好地模仿天然胶原组织的结构并实现预期的机械性能和生理功能。 丝素蛋白作为一种多功能蛋白质生物材料常用于形成薄膜、纤维、凝胶微球和多孔支架等。许多研究表明,丝素蛋白电凝胶主要由无规卷曲和α-螺旋构象的丝素蛋白组成[59-60],这种凝胶的自组装是可逆的,在电加工体系中丝素蛋白在阳极附近形成凝胶,当反转电极极性时凝胶溶解并在另一电极上重新形成凝胶[61]。通过电加工可以在具有复杂几何形状的电极表面形成丝素蛋白薄膜,薄膜形成的速度和厚度可以通过电压大小、沉积时间和沉积液浓度来调节[41,59,62]。SERVOLI等[63]的研究表明,在交流电场下浇注丝素蛋白溶液时可产生各向异性和水中稳定的薄膜,这些作用与分子偶极的排列和定向的超分子组装有关。电加工在某些情况下(与pH值局部变化有关)可引导蛋白质构象由无定形向β-折叠转变,赋予薄膜更好的机械和热稳定性[41]。还可以对电凝胶二次加工(例如进行局部化学交联)调控其结构,如LIN等[64]在研究中使用戊二醛处理丝素蛋白电凝胶,诱导蛋白质构象向β-折叠态转变,从而提高其物理机械性能。 对于蛋白质的电加工,人们在研究时更注重其具体应用,比如作为支架材料、药物载体或者体内植入体的涂层等[65-66],对材料沉积动力学相关的研究较少。"

| [1] GROLL J, BOLAND T, BLUNK T, et al. Biofabrication:reappraising the definition of an evolving field.Biofabrication.2016;8(1):013001. [2] MIRONOV V, TRUSK T, KASYANOV V, et al. Biofabrication:a 21st century manufacturing paradigm.Biofabrication.2009;1(2):022001. [3] LI Y, XIAO Y, LIU C. The Horizon of Materiobiology:A Perspective on Material-Guided Cell Behaviors and Tissue Engineering.Chem Rev.2017;117(5): 4376-4421. [4] ROGAN H, ILAGAN F, TONG XM, et al. Microribbon-hydrogel composite scaffold accelerates cartilage regeneration in vivo with enhanced mechanical properties using mixed stem cells and chondrocytes.Biomaterials. 2020;228: 10. [5] CHANTRE CO, GONZALEZ GM, AHN S, et al. Porous Biomimetic Hyaluronic Acid and Extracellular Matrix Protein Nanofiber Scaffolds for Accelerated Cutaneous Tissue Repair.ACS Appl Mater Interfaces.2019;11(49): 45498-45510. [6] TAMAYOL A, AKBARI M, ANNABI N, et al. Fiber-based tissue engineering: Progress, challenges,and opportunities.Biotechnol Adv.2013;31(5):669-687. [7] CHEN Q, WU J, LIU Y, et al. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing.Mater Sci Eng,C.2019;105:110083. [8] GALARRAGA JH, KWON MY, BURDICK JA. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue.Sci Rep.2019; 9(1):19987. [9] FRIGUGLIETTI J, DAS S, LE P, et al. Novel Silicon Titanium Diboride Micropatterned Substrates for Cellular Patterning. Biomaterials.2020;244: 119927. [10] SUN X, TYAGI P, AGATE S, et al. Highly tunable bioadhesion and optics of 3D printable PNIPAm/cellulose nanofibrils hydrogels.Carbohydr Polym. 2020; 234:115898. [11] SHEN LB, GAO N, HUANG XR. Synthesis, characterization and electropolymerization of functionalized organic salt-anilinium saccharinate and electrochemically controlled release of saccharinate anions.Electrochim Acta. 2020;329:8. [12] ODIJK M, VAN DEN BERG A. Nanoscale Electrochemical Sensing and Processing in Microreactors.Annu Rev Anal Chem.2018;11:421-440. [13] FEIG VR, TRAN H, LEE M, et al. An Electrochemical Gelation Method for Patterning Conductive PEDOT:PSS Hydrogels.Adv Mater.2019;31(39): e1902869. [14] QIU K, CHEN B, NIE W, et al. Electrophoretic Deposition of Dexamethasone-Loaded Mesoporous Silica Nanoparticles onto Poly(l-Lactic Acid)/Poly(ε-Caprolactone) Composite Scaffold for Bone Tissue Engineering.ACS Appl Mater Interfaces.2016;8(6):4137-4148. [15] QU X, LIU H, ZHANG C, et al.E lectrofabrication of functional materials: Chloramine-based antimicrobial film for infectious wound treatment.Acta Biomater.2018;73:190-203. [16] WANG X, YAN L, YE T, et al. Osteogenic and antiseptic nanocoating by in situ chitosan regulated electrochemical deposition for promoting osseointegration. Mater Sci Eng,C.2019;102:415-426. [17] GARGAVA A, AHN S, BENTLEY WE, et al. Rapid Electroformation of Biopolymer Gels in Prescribed Shapes and Patterns:A Simpler Alternative to 3-D Printing.ACS Appl Mater Interfaces.2019;11(40):37103-37111. [18] MAERTEN C, JIERRY L, SCHAAF P, et al. Review of Electrochemically Triggered Macromolecular Film Buildup Processes and Their Biomedical Applications.ACS Appl Mater Interfaces.2017;9(34):28117-28138. [19] THOMAS MB, METOKI N, MANDLER D, et al. In Situ Potentiostatic Deposition of Calcium Phosphate with Gentamicin-Loaded Chitosan Nanoparticles on Titanium Alloy Surfaces.Electrochim Acta.2016;222:355-360. [20] GEULI O, METOKI N, ELIAZ N, et al. Electrochemically Driven Hydroxyapatite Nanoparticles Coating of Medical Implants.Adv Funct Mater.2016;26(44): 8003-8010. [21] HAN C, YAO Y, CHENG X, et al. Electrophoretic Deposition of Gentamicin-Loaded Silk Fibroin Coatings on 3D-Printed Porous Cobalt-Chromium-Molybdenum Bone Substitutes to Prevent Orthopedic Implant Infections.Biomacromolecules.2017;18(11):3776-3787. [22] LI J, MANIAR D, QU X, et al. Coupling Self-Assembly Mechanisms to Fabricate Molecularly and Electrically Responsive Films.Biomacromolecules. 2019; 20(2):969-978. [23] LI C, BAI H, SHI G. Conducting polymer nanomaterials:electrosynthesis and applications.Chem Soc Rev.2009;38(8):2397-2409. [24] SADMAN K, WANG Q, CHEN SH, et al. pH-Controlled Electrochemical Deposition of Polyelectrolyte Complex Films.Langmuir.2017;33(8):1834-1844. [25] MARTIN EJ, SADMAN K, SHULL KR. Anodic Electrodeposition of a Cationic Polyelectrolyte in the Presence of Multivalent Anions.Langmuir.2016;32(31): 7747-7756. [26] CROSS ER. The electrochemical fabrication of hydrogels:a short review.SN Appl Sci.2020;2(3):397. [27] REDEPENNING J, VENKATARAMAN G, CHEN J, et al. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates.J Biomed Mater Res,Part A.2003;66A(2):411-416. [28] WU LQ, GADRE AP, YI H, et al. Voltage-Dependent Assembly of the Polysaccharide Chitosan onto an Electrode Surface.Langmuir.2002;18(22): 8620-8625. [29] YOKOYAMA F, FUJINO T, KIMURA K, et al. Formation of optically anisotropic alginic acid gels under dc electric fields.Eur Polym J.1998;34(2):229-234. [30] CHEONG M, ZHITOMIRSKY I. Electrodeposition of alginic acid and composite films.Colloids Surf Physicochem Eng Aspects.2008;328(1):73-78. [31] BRUCHET M, MELMAN A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange.Carbohydr Polym.2015;131:57-64. [32] GENG Z, WANG X, GUO X, et al. Electrodeposition of chitosan based on coordination with metal ions in situ-generated by electrochemical oxidation.J Mater Chem B.2016;4(19):3331-3338. [33] YAN K, DING F, BENTLEY WE, et al. Coding for hydrogel organization through signal guided self-assembly.Soft Matter.2014;10(3):465-469. [34] LING T, LIN J, TU J, et al. Mineralized collagen coatings formed by electrochemical deposition.J Mater Sci Mater Med.2013;24(12):2709-2718. [35] BESRA L, LIU M. A review on fundamentals and applications of electrophoretic deposition (EPD).Prog Mater Sci.2007;52(1):1-61. [36] MARTIN LJ, AKHAVAN B, BILEK MMM. Electric fields control the orientation of peptides irreversibly immobilized on radical-functionalized surfaces.Nat Commun.2018;9(1):357. [37] LEI M, QU X, LIU H, et al. Programmable Electrofabrication of Porous Janus Films with Tunable Janus Balance for Anisotropic Cell Guidance and Tissue Regeneration.Adv Funct Mater.2019;29(18):1900065. [38] WU S, YAN K, ZHAO Y, et al. Electrical Writing onto a Dynamically Responsive Polysaccharide Medium:Patterning Structure and Function into a Reconfigurable Medium.Adv Funct Mater.2018;28(40):1803139. [39] YI H, WU LQ, BENTLEY WE, et al. Biofabrication with Chitosan.Biomacromolecules. 2005;6(6):2881-2894. [40] RAEBURN J, ALSTON B, KROEGER J, et al. Electrochemically-triggered spatially and temporally resolved multi-component gels.Mater Horiz.2014; 1(2):241-246. [41] BRESSNER JE, MARELLI B, QIN G, et al. Rapid fabrication of silk films with controlled architectures via electrogelation.J Mater Chem B.2014;2(31): 4983-4987. [42] YAN K, LIU Y, ZHANG J, et al. Electrical Programming of Soft Matter:Using Temporally Varying Electrical Inputs To Spatially Control Self Assembly.Biomacromolecules.2018;19(2):364-373. [43] LIU Y, ZHANG B, GRAY KM, et al. Electrodeposition of a weak polyelectrolyte hydrogel:remarkable effects of salt on kinetics,structure and properties.Soft Matter.2013;9(9):2703-2710. [44] TSAI C-C, MORROW BH, CHEN W, et al. Toward Understanding the Environmental Control of Hydrogel Film Properties:How Salt Modulates the Flexibility of Chitosan Chains.Macromolecules.2017;50(15):5946-5952. [45] SIMCHI A, PISHBIN F, BOCCACCINI AR. Electrophoretic deposition of chitosan. Mater Lett.2009;63(26):2253-2256. [46] CHENG Y, LUO X, BETZ J, et al. In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes. Soft Matter.2010;6(14):3177-3183. [47] NING C, JIAJIA J, MENG L, et al. Electrophoretic deposition of GHK-Cu loaded MSN-chitosan coatings with pH-responsive release of copper and its bioactivity.Mater Sci Eng C.2019;104:109746. [48] GITERU SG, OEY I, ALI MA. Feasibility of using pulsed electric fields to modify biomacromolecules:A review.Trends Food Sci Technol.2018;72:91-113. [49] GRAY KM, LIBA BD, WANG Y, et al. Electrodeposition of a Biopolymeric Hydrogel: Potential for One-Step Protein Electroaddressing.Biomacromolecules. 2012;13(4):1181-1189. [50] LEE KY, MOONEY DJ. Alginate:Properties and biomedical applications.Prog Polym Sci.2012;37(1):106-126. [51] JIN Z, GüVEN G, BOCHAROVA V, et al. Electrochemically Controlled Drug-Mimicking Protein Release from Iron-Alginate Thin-Films Associated with an Electrode.ACS Appl Mater Interfaces.2012;4(1):466-475. [52] CHENG Y, LUO X, BETZ J, et al. Mechanism of anodic electrodeposition of calcium alginate.Soft Matter.2011;7(12):5677-5684. [53] TAIRA N, INO K, ROBERT J, et al. Electrochemical printing of calcium alginate/gelatin hydrogel.Electrochim Acta.2018;281:429-436. [54] BAKER HR, MERSCHROD S EF, PODUSKA KM. Electrochemically Controlled Growth and Positioning of Suspended Collagen Membranes.Langmuir. 2008; 24(7):2970-2972. [55] DEWLE A, PATHAK N, RAKSHASMARE P, et al. Multifarious Fabrication Approaches of Producing Aligned Collagen Scaffolds for Tissue Engineering Applications.ACS Biomater Sci Eng.2020;6(2):779-797. [56] BARRETT DJ, LINLEY MD, BEST SM, et al. Fabrication of free standing collagen membranes by pulsed-electrophoretic deposition.Biofabrication. 2019;11(4):045017. [57] CHENG X, GURKAN UA, DEHEN CJ, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles.Biomaterials.2008;29(22):3278-3288. [58] UQUILLAS JA, KISHORE V, AKKUS O. Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads. Biomed Mater. 2011;6(3):035008. [59] ZHANG Q, HAN G, LU C, et al. Facile Preparation of Mechanical Reinforced and Biocompatible Silk Gels.Fibers Polym.2019;20(4):675-682. [60] LU Q, HUANG Y, LI M, et al. Silk fibroin electrogelation mechanisms.Acta Biomater.2011;7(6):2394-2400. [61] LEISK GG, LO TJ, YUCEL T, et al. Electrogelation for Protein Adhesives.Adv Mater.2010;22(6):711-715. [62] GUO YQ, GUAN J, PENG H, et al. Tightly adhered silk fibroin coatings on Ti6Al4V biometals for improved wettability and compatible mechanical properties.Mater Des.2019;175:9. [63] SERVOLI E, MANIGLIO D, MOTTA A, et al. Folding and Assembly of Fibroin Driven by an AC Electric Field:Effects on Film Properties.Macromol Biosci. 2008;8(9):827-835. [64] LIN Y, XIA X, SHANG K, et al. Tuning Chemical and Physical Cross-Links in Silk Electrogels for Morphological Analysis and Mechanical Reinforcement.Biomacromolecules.2013;14(8):2629-2635. [65] SAHA S, PRAMANIK K, BISWAS A. Silk fibroin coated TiO2 nanotubes for improved osteogenic property of Ti6Al4V bone implants.Mater Sci Eng, C. 2019;105:14. [66] KARAHALILOĞLU Z. Curcumin-loaded silk fibroin e-gel scaffolds for wound healing applications.Mater Technol.2018;33(4):276-287. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [3] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [4] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [5] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [6] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [7] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [8] | Jiang Hongying, Zhu Liang, Yu Xi, Huang Jing, Xiang Xiaona, Lan Zhengyan, He Hongchen. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1149-1153. |
| [9] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [10] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [11] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [12] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [13] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [14] | Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling. Photoreceptor cell replacement therapy for retinal degeneration diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1116-1121. |
| [15] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||