Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (4): 612-618.doi: 10.3969/j.issn.2095-4344.1938
Previous Articles Next Articles
Specific bone-targeting nanoscale drug delivery system: advantages and clinical applicability
Xiang Haibin1, Li Xinxia2, Liang Qiuzhen1, Song Xinghua1
- 1Department of Orthopedics, the First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China; 2College of Pharmacy, Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China
-
Received:2019-04-08Revised:2019-04-19Accepted:2019-05-31Online:2020-02-08Published:2020-01-07 -
Contact:Song Xinghua, Chief physician, Professor, Doctor’s supervisor, Department of Orthopedics, the First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China -
About author:Xiang Haibin, Master candidate, Department of Orthopedics, the First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81860394
CLC Number:
Cite this article
Xiang Haibin, Li Xinxia, Liang Qiuzhen, Song Xinghua. Specific bone-targeting nanoscale drug delivery system: advantages and clinical applicability [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 612-618.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

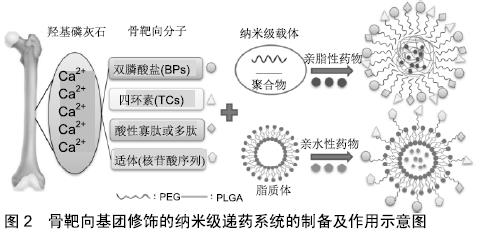
2.1 主动骨靶向递药系统的机制 目前药物主动靶向递送至骨骼系统的方法有2种,其一以骨的特定细胞为靶点,通常是破骨细胞、成骨细胞、骨髓间充质干细胞;其二则是以整个骨组织为靶点,在正常骨骼中有机基约占30%,无机基质占65%-70%,骨细胞仅占总干骨质量的1%-2%,而其中的无机基质主要是由羟基磷灰石组成[30]。目前基于骨羟基磷灰石相应骨细胞的靶向分子包括蛋白质(主要是抗体及补体)、多肽、核酸、小分子化合物[31]。 2.2 骨靶向分子 2.2.1 双膦酸盐 双膦酸盐是一类合成药物,首次被报道于1969年,目前已被应用于骨质疏松症、骨肿瘤溶骨性破坏引起的疼痛及高钙血症、代谢性骨病近40年[32]。其基本结构含有2个共有1个碳原子的膦酸酯基团,也称为“P-C-P”骨架。相比天然存在的具有“P-O-P”结构的焦磷酸盐来说,双膦酸盐则更加稳定,同时保持其对骨矿物质的亲和力而长期停留[33]。而它的骨靶向性机制为:双膦酸盐上的2个膦酸酯基团中的去质子化羟基与羟基磷灰石上的二价钙离子(Ca2+)螯合形成双齿样结构[34]。当去质子化的羟基基团之间的距离增加如P-N-P、P-C-C-P骨架的化合物或者单磷酸盐都会对羟基磷灰石的结合亲和力降低,而“P-C-P”骨架碳原子上的2个剩余基团R1和R2也可进一步调节对羟基磷灰石的亲和力,如R1处存在羟基(-OH)或胺基(-NH2)与钙离子的额外相互作用对羟基磷灰石更具有亲和力[35-36]。对于6种不同的二膦酸盐与羟基磷灰石亲和力排名如下:唑来膦酸盐>阿仑膦酸盐>伊班膦酸盐>利塞膦酸盐>依替膦酸盐>氯膦酸盐[37-38]。此外,双膦酸盐上的-OH或-NH2基团可用于化学修饰,例如与药物缀合形成前药或缀合载体形成纳米粒,而不损害他们对骨骼的亲和 力[31,34]。但在极少数情况下,双膦酸盐的长期和过度使用与血压升高与股骨骨折以及颌骨坏死相关[39]。 2.2.2 四环素 四环素是由链霉菌和金黄色链霉菌菌株产生的小分子化合物,传统上用于治疗细菌感染的抗生素,近年来也显示出作为骨靶向部分的前景。四环素分子结构式中的位置1、2处β二酮,4、6处的烯醇及5处甲酰胺基团可以与羟基磷灰石上Ca2+螯合[40]。NEALE等[41]改造四环素的A环中的三羰基甲烷基团所得的3-氨基-2,6-二羟基苯甲酰胺环结构对羟基磷灰石的结合亲和力增加高达50%。除螯合作用外,四环素分子的羟基与磷灰石之间分子范德华力和氢键也被认为提供了额外的相互作用。此外还报道了雌二醇直接缀合四环素 环A,增加了卵巢切除大鼠骨骼组织中雌二醇的积累而不增加子宫中的积累。但由于四环素对骨螯合作用是永久的,所以会引起儿童的牙齿染色及其不良反应,包括光敏性、皮疹、胃灼热、低血糖儿童头晕、恶心[42]。 2.2.3 肽类 寡肽中的天冬氨酸(Asp)或谷氨酸(Glu)对于羟基磷灰石具有亲和力[43-44],尽管确切机制没有统一,但可以明确的是因为骨基质蛋白如骨桥蛋白和骨钙蛋白的存在,使得氨基酸序列中存在天冬氨酸或谷氨酸的重复单元时,对羟基磷灰石的亲和力增加[45]。 天冬氨酸多肽(Asp):TAKAHASHI等[46]2008年以天冬氨酸L-(Asp)6共轭喹诺酮类药物用于骨髓炎小鼠模型中,L-Asp6共轭的喹诺酮选择性地分布到骨的浓度是非共轭喹诺酮100倍,同时持续至注射后至少6 d。JIANG等[27]2014年用异硫氰酸荧光素(FITC)标记的寡聚物(FITC-Asp7Cys)结合聚乙二醇-聚乳酸-羟基乙酸颗粒形成纳米粒,在体外羟基磷灰石亲和力实验中FITC标记颗粒的上清液荧光量从100%减少至20%,这表明与羟基磷灰石的强烈且特异性相互作用,且在小鼠全身给药中骨组织优先积累而其他组织受限。ZHANG等[47]制备了骨靶向的D-Asp8-N-(2-羟丙基)甲基丙烯酰胺聚合物纳米粒子,以递送siRNA分子干扰Semaphorin 4D (SEMA4D)表达来特异性针对破骨细胞,同时在卵巢切除的动物模型中与非靶向的对照组相比,在注射后的破骨细胞中观察到SEMA4D表达降低超过4倍。 天冬氨酸-丝氨酸-丝氨酸:天冬氨酸-丝氨酸-丝氨酸是第一个设计用于靶向牙本质磷蛋白的肽,对骨形成表面的低结晶羟基磷灰石具有高亲和力,这是成骨细胞的物理特性。ZHANG等[48]将调节Plekho1基因的siRNA包封在DOTAP(二油酰基三甲基铵丙烷)阳离子脂质体递送系统中,其脂质体表面与(AspSerSer)6肽部分共价连接用于靶向成骨细胞,它通过递送siRNA以下调CKIP-1蛋白的表达,在大鼠中全身给药中荧光标记的(天冬氨酸SerSer)6脂质体导致骨中的强荧光信号,但在其他器官中几乎没有。相反,没有靶向肽的DOTAP脂质体在骨中具有较弱的荧光信号。 其他肽序列:环状精氨酸-甘氨酸-天冬氨酸-酪氨酸-赖氨酸(cRGDyk)可通过选择性抑制骨中富含αvβ3整合素的肿瘤细胞。WANG等[49]开发了缀合cRGDyk的纳米粒包封顺铂,以改善前列腺癌骨转移鼠模型中的细胞摄取和抗肿瘤效率。与用脂质体包封药物处理的小鼠相比,该cRGDyk肽缀合的脂质体药物递送系统在体外对鼠前列腺癌细胞具有高3倍的细胞摄取和更高的细胞毒性。随着噬菌体表面呈现技术的发展,SUN等[21]开发了丝氨酸-天冬氨酸-丝氨酸-丝氨酸-丝氨酸结合聚氨酯(PU)纳米胶束包裹siRNA和miRNA靶向成骨细胞,并通过DMP1(成骨细胞标记物)和荧光标记的FAM-siRNA证实了它的靶向性。此外还有3种具有序列VTKHLNQISQSY(VTK),STLPIPHEFSRE和APWHLSSQYSRT对羟基磷灰石和骨样材料具有强烈且特异的亲和力[50-51]。 2.2.4 适体 适体是一类短的单链DNA或RNA核苷酸序列(25-35碱基长),当折叠成其独特的三级构象时,可以高特异性识别并结合其靶标。它们的特异性来源于指数富集配体的系统进化(SELEX)方法,用于纯化适体配体特异性结合[52]。LIANG等[26]用成骨细胞特异性适体CH6与脂质体纳米粒结合来递送siRNA以下调CKIP-1,与非靶向的脂质体纳米粒LNP相比,CH6-LNP-siRNA在骨组织中显示更高的积累并且在大鼠肝和肾中积累更低。用CH6-LNP-siRNA处理的去卵巢大鼠具有改善的骨微结构,增加的骨量和增强的机械性质而没有不良反应。此外与CH2和CH5相比,CH6适体具有更短的核苷酸序列和更好二级结构。 2.3 骨靶向递药系统中的纳米级载体 近年来,纳米科学和纳米技术领域的研究和应用正在飞速的发展。纳米载体可以改善药物溶解度、控制释放速率、保护药物免受酶促降解、延长循环时间并降低药物的毒性[53]。用于载药的纳米粒有:二氧化硅米粒、钛、纳米管、金纳米粒、磷酸钙纳米粒等无机纳米粒,还有壳聚糖纳米粒、聚合物纳米粒、脂质纳米粒等有机纳米粒。在骨靶向递药系统中,前者较常用于局部给药而后者常用于全身给药。聚合物载体因其相对容易制造和可调节的物理性质而被广泛使用,如聚乳酸-羟基乙酸、N-(2-羟丙基)甲基丙烯酰胺、聚乳酸[54]。 聚乳酸-羟基乙酸纳米粒是应用最为广泛的聚合物载体,因其水解产生代谢物为乳酸和乙醇酸可以通过Krebs循环容易被身体代谢,因此也是全身毒性最小载体[55]。其次聚乳酸-羟基乙酸纳米粒子的药物释放速率也可以通过控制乳酸和乙醇酸单体比例及聚合物的分子质量来调节[56]。目前聚乳酸-羟基乙酸已经正式被美国FDA和欧洲医药机构批准用于人类的各种药物输送系统。WANG等[22]报道了用四环素修饰的聚乳酸-羟基乙酸包裹辛伐他汀作为骨质疏松症新型治疗手段。 但是聚乳酸-羟基乙酸纳米粒在血液中循环中很容易被调理素的血清蛋白识别,使得纳米粒容易被单核吞噬细胞系统吞噬,从而加快药物代谢。此外纳米粒也可直接由巨噬细胞通过调理素无关的清道夫受体捕获[57]。聚乙二醇是一种常见“隐形”聚合物,可通过简单的有机合成来修饰纳米颗粒,使其逃避免疫系统的检测,以延长循环和减少巨噬细胞摄取。这种经过“隐形”聚合修饰的纳米粒,它具有独特核壳结构,外壳一般是亲水性聚合物,内核一般是疏水性聚合物并包裹脂溶性药物。但有时也可由亲水性聚合物通过化学键结合难溶性药物,再通过由2个带相反电荷的片段以聚离子复合物方式形成胶束[58]。它的优点有:具有热力学稳定;可包封疏水或亲水药物,尤其是难溶性药物可包裹在疏水核中;通常粒径介于10-100 nm,因此容易通过高渗透长滞留效应靶向到肿瘤组织;长循环效应,因为聚乙二醇化的共聚物通过避免网状内皮系统的吸收;良好的聚合物降解和生物相容性。聚合物胶束除了最常见的两嵌段共聚物外,三嵌段共聚物和接枝共聚物也常有研究,如聚乳酸-羟基乙酸-聚乙二醇-聚乳酸-羟基乙酸,聚乙二醇-聚乳酸-羟基乙酸-聚乙二醇被用于温度敏感型靶向载体[59]。这些聚合物也可通过结合骨靶向分子来包裹药物形成特异性骨靶向纳米递药系统,见图2。 "

2.4 特异性骨靶向纳米级递药系统的应用 2.4.1 骨髓炎 可能是由菌血症后的血源性扩散引起的急性骨髓炎,也可能是未经治疗或治疗失败的开放性骨折、菌血症或连续软组织感染所致的慢性骨髓炎。金黄色葡萄球菌是骨髓炎中的主要致病菌。通常慢性骨髓炎治疗的金标准是坏死骨和周围感染组织的外科清创术,然后进行数周的全身抗生素治疗。而抗生素浓度必须长期保持在非常高的水平,这样会增加不良反应的风险。因此负载抗生素的聚甲基丙烯酸甲酯已被应用了数十年,但随之也出现了大量问题。聚甲基丙烯酸甲酯的热聚合特性使得只能负载有限数量的热稳定抗生素,并且其负载药物含量释放不完全[60]。目前已报道了许多用骨靶向分子直接缀合抗生素的前药,其目的是减少全身剂量并增加骨组织抗生素浓度。TAKAHASHI等[46]将左氧氟沙星和诺氟沙星分别与天冬氨酸六肽缀合形成Asp6-左氧氟沙星和Asp6-诺氟沙星,与游离喹诺酮相比,其对羟基磷灰石的体外亲和力增加,但Asp6-左氧氟沙星没有显示出相似的特性。在正常小鼠中,在1周内Asp6-左氧氟沙星比未缀合的左氧氟沙星在骨和骨髓上的浓度更高;在骨髓炎小鼠模型中,Asp6-左氧氟沙星选择性地分布到骨的浓度是非共轭喹诺酮的100倍,同时持续至注射后至少6 d。此外也报道了双膦酸盐缀合的许多抗生素包括诺氟沙星、加替沙星、莫西沙星、氟喹诺酮酯、利福拉齐、环丙沙星,在最近一次SEDGHIZADEH等[34,61]的报道中单剂量的10 mg/kg的双膦酸盐-环丙沙星共价结合物显示动物骨髓炎模型中细菌负荷减少99%。然而前药仍然存在释放时间的问题,如果靶向基团和药物共价结合过于稳定而不利于药物的释放,但如果共价键不稳定又可能在药物达到骨组织之前提前释放,亦不能得到很好的靶向性。因此FERREIRA等开发了阿仑膦酸盐结合的脂质体来负载(99Tcm)放射性标记的头孢唑肟用于骨髓炎的诊断和治疗,并通过骨扫描证明了Tc-放射性标记的头孢唑肟增加了胫骨中的靶-非靶比率。 2.4.2 骨肉瘤及骨转移癌 骨肉瘤是较为常见的原发性骨肿瘤,起源于间充质组织,70%-80%患者年龄在10- 25岁[62]。而骨是晚期癌症中最常见的实体肿瘤转移器官之一,70%乳腺癌及前列腺癌主要转移至骨。目前骨肉瘤和骨转移癌治疗的金标准为手术切除肿瘤联合化学治疗,而用于化疗药物的主要有多柔比星、甲氨蝶呤、顺铂和异环磷酰胺。尽管这些可阻止肿瘤细胞增殖,但药物不区分肿瘤细胞和健康细胞,导致严重的全身不良反应。LIU等[15]合成了用天冬氨酸八肽作为靶向基团共价结合聚合物载体聚乙二醇-聚己内酯包裹姜黄素治疗乳腺癌骨转移,并证明了Asp8-聚乙二醇-聚己内酯纳米颗粒对MG63和人脐静脉内皮细胞没有细胞毒作用。用于乳腺癌骨转移的小鼠模型中,与聚乙二醇-聚己内酯相比静脉内注射Asp8-聚乙二醇-聚己内酯纳米颗粒在小鼠胫骨上累积多2.7倍,且包裹姜黄素Asp8-聚乙二醇-聚己内酯纳米颗粒以高剂量持续释放姜黄素>8 d。Yin等[18]报道了帕米膦酸盐缀合聚乳酸纳米颗粒Pam-PLA负载多柔比星用于狗及小鼠的骨肉瘤模型。在局灶性恶性骨溶解的小鼠模型中,载有的多柔比星的Pam-PLA纳米颗粒显着控制了局部骨肉瘤进展,并用99Tcm标记载有多柔比星的Pam-PLA纳米颗粒用于骨肉瘤模型显示优先在溶骨病变区蓄积。 2.4.3 多发性骨髓瘤 多发性骨髓瘤是一种血液系统恶性肿瘤,由骨髓中肿瘤浆细胞的积聚和增殖引起,其不可治愈与复发率大于90%。目前被用于多发性骨髓瘤化疗药物有蒽环类抗生素(多柔比星)、糖皮质激素(地塞米松和泼尼松)和氮芥烷基化剂及一些新药如免疫调节药物沙利度胺、蛋白酶抑制剂硼替佐米[63]。硼替佐米和卡非佐米的使用明显改善了多发性骨髓瘤患者的存活率,但硼替佐米受其神经毒性的限制,尤其是周围神经导致感觉轴突神经变性[64]。SWAMI等[28]用阿仑膦酸盐缀合聚乙二醇-聚乳酸-羟基乙酸负载硼替佐米,通过纳米沉淀法制备出粒径75 nm纳米粒,在正常小鼠模型中腹膜内注射24 h后,用流式细胞术测定在脾脏、股骨和颅骨中纳米粒含量增加,与非靶向纳米粒相比靶向性增加约9倍。 2.4.4 骨质疏松症 骨质疏松症是一种常见的骨代谢性疾病,目前一半以上的患者正在接受药物治疗,而且随着人口老年化骨质疏松症可能变得更加普遍。从出生到二三十岁骨质量逐年增加,骨形成的成骨细胞的活性超过骨吸收的破骨细胞的活性。但经过成年期骨密度峰值之后,破骨细胞的活性开始超过成骨细胞,可能导致骨质疏松,随之而来的骨痛及骨折风险也相应增加。目前针对于骨质疏松症主要有抗骨吸收及控制合成代谢药物来抑制破骨细胞的发育和活性,如雌激素、雌激素衍生物、双膦酸盐,降钙素、Denosumab和甲状旁腺激素等[65]。虽然这些药物均取得了良好的抗骨质疏松效果,但仍然存在患者不依从或生物利用度差的缺点,随之一些毒副作用也被相继报道,如雌激素相关的乳腺癌、子宫内膜癌、血栓和心脏病的风险增加。在最近一项研究中,XIE等[12]利用四环素作为靶向基团缀合了聚乙二醇-聚乳酸-羟基乙酸形成聚合物纳米粒负载辛伐他汀作为骨质疏松症得新型治疗手段。从体外骨矿物质结合能力来说,四环素-聚乙二醇-聚乳酸-羟基乙酸纳米粒与羟基磷灰石结合率较聚乙二醇-聚乳酸-羟基乙酸纳米粒高(79.5%,20.1%,P < 0.05),从而证明了四环素对羟基磷灰石具有较强的亲和力。此外经小鼠尾部静脉注射四环素-聚乙二醇-聚乳酸-羟基乙酸纳米粒24 h后,四环素修饰的纳米粒在小鼠的骨骼组织显示强荧光。 "

| [1] PIERCE WM, WAITE LC.Bone-targeted carbonic anhydrase inhibitors: effect of a proinhibitor on bone resorption in vitro.Proc Soc Exp Biol Med.1987;186(1):96-102. [2] GU W, WU C, CHEN J, et al. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Nanomedicine. 2013;8:2305-2317. [3] CHEN J, DING J, ZHANG Y, et al.Polyion complex micelles with gradient pH-sensitivity for adjustable intracellular drug delivery.Polym Chem. 2015;6(3):397-405. [4] NEGUSSIE AH, MILLER JL, REDDY G, et al.Synthesisand and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J Control Release.2010;143(2):265-273. [5] CHEN B, YANG JZ, WANG LF, et al.Ifosfamide-loaded poly (lactic-co-glycolic acid) PLGA-dextran polymeric nanoparticles to improve the antitumor efficacy in Osteosarcoma.BMC Cancer. 2015;15:752. [6] ABRAHAMSE H, KRUGER CA, KADANYO S, et al.Nanoparticles for Advanced Photodynamic Therapy of Cancer.Photomed Laser Surg. 2017;35(11):581-588. [7] DANG J, HE H, CHEN D, et al.Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT).Biomater Sci.2017;5(8): 1500-1511. [8] MAEDA H.Polymer therapeutics and the EPR effect.J Drug Target. 2017;25(9-10):781-785. [9] WANG Y, LIU Z, LI T, et al.Enhanced Therapeutic Effect of RGD-Modified Polymeric Micelles Loaded With Low-Dose Methotrexate and Nimesulide on Rheumatoid Arthritis. Theranostics. 2019;9(3):708-720. [10] HUANG L, WANG X, CAO H, et al.A bone-targeting delivery system carrying osteogenic phytomolecule icaritin prevents osteoporosis in mice.Biomaterials.2018;182:58-71. [11] YAMASHITA S, KATSUMI H, HIBINO N, et al.Development of PEGylated aspartic acid-modified liposome as a bone-targeting carrier for the delivery of paclitaxel and treatment of bone metastasis. Biomaterials. 2018;154:74-85. [12] XIE Y, LIU C, HUANG H, et al.Bone-targeted delivery of simvastatin- loaded PEG-PLGA micelles conjugated with tetracycline for osteoporosis treatment.Drug Deliv Transl Res.2018;8(5):1090-1102. [13] KE X, LIN W, LI X, et al.Synergistic dual-modified liposome improves targeting and therapeutic efficacy of bone metastasis from breast cancer.Drug Deliv.2017;24(1):1680-1689. [14] HAGHIRALSADAT F, AMOABEDINY G, NADERINEZHAD S, et al. EphA2 Targeted Doxorubicin-Nanoliposomes for Osteosarcoma Treatment.Pharm Res. 2017;34(12):2891-2900. [15] LIU J, ZENG Y, SHI S, et al.Design of polyaspartic acid peptide-poly (ethylene glycol)-poly (epsilon-caprolactone) nanoparticles as a carrier of hydrophobic drugs targeting cancer metastasized to bone.Int J Nanomedicine.2017;12:3561-3575. [16] RAICHUR V, VEMULA KD, BHADRI N, et al.Zolendronic Acid-Conjugated PLGA Ultrasmall Nanoparticle Loaded with Methotrexate as a Supercarrier for Bone-Targeted Drug Delivery.AAPS Pharm Sci Tech. 2017;18(6):2227-2239. [17] HE Y, HUANG Y, HUANG Z, et al.Bisphosphonate-functionalized coordination polymer nanoparticles for the treatment of bone metastatic breast cancer.J Control Release.2017;264:76-88. [18] YIN Q, TANG L, CAI K, et al.Pamidronate functionalized nanoconjugates for targeted therapy of focal skeletal malignant osteolysis.Proc Natl Acad Sci U S A.2016;113(32):E4601-4609. [19] CHANG Q, GENG R, WANG S, et al.DOPA-based paclitaxel-loaded liposomes with modifications of transferrin and alendronate for bone and myeloma targeting.Drug Deliv.2016;23(9):3629-3638. [20] SUN W, HAN Y, LI Z, et al.Bone-Targeted Mesoporous Silica Nanocarrier Anchored by Zoledronate for Cancer Bone Metastasis. Langmuir. 2016;32(36):9237-9244. [21] SUN Y, YE X, CAI M, et al.Osteoblast-Targeting-Peptide Modified Nanoparticle for siRNA/microRNA Delivery.ACS Nano. 2016;10(6): 5759-5768. [22] WANG H, LIU J, TAO S, et al.Tetracycline-grafted PLGA nanoparticles as bone-targeting drug delivery system.Int J Nanomedicine. 2015;10: 5671-5685. [23] FERREIRA DDOS S, BORATTO FA, CARDOSO VN, et al. Alendronate-coated long-circulating liposomes containing 99mtechnetium-ceftizoxime used to identify osteomyelitis.Int J Nanomedicine.2015;10:2441-2450. [24] CONG Y, QUAN C, LIU M, et al.Alendronate-decorated biodegradable polymeric micelles for potential bone-targeted delivery of vancomycin. J Biomater Sci Polym Ed.2015;26(11):629-643. [25] LIU J, DANG L, LI D, et al.A delivery system specifically approaching bone resorption surfaces to facilitate therapeutic modulation of microRNAs in osteoclasts.Biomaterials.2015;52:148-160. [26] LIANG C, GUO B, WU H, et al.Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy.Nat Med.2015;21(3):288-294. [27] JIANG T, YU X, CARBONE EJ, et al.Poly aspartic acid peptide-linked PLGA based nanoscale particles: potential for bone-targeting drug delivery applications.Int J Pharm.2014;475(1-2):547-557. [28] SWAMI A, REAGAN MR, BASTO P, et al.Engineered nanomedicine for myeloma and bone microenvironment targeting.Proc Natl Acad Sci U S A. 2014;111(28):10287-10292. [29] WANG F, CHEN L, ZHANG R, et al.RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer.J Control Release.2014;196:222-233. [30] CLARKE B.Normal bone anatomy and physiology.Am Soc Nephrol. 2008;3(Suppl 3):S131-139. [31] CARBONE EJ, RAJPURA K, ALLEN BN,e t al.Osteotropic nanoscale drug delivery systems based on small molecule bone-targeting moieties. Nanomedicine.2017;13(1):37-47. [32] RUSSELL RG.Bisphosphonates: The first 40 years.Bone. 2011;49(1): 2-19. [33] BROWN JP, MORIN S, LESLIE W, et al.Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician.2014;60(4):324-333. [34] FARRELL KB, KARPEISKY A,THAMM DH, et al.Bisphosphonate conjugation for bone specific drug targeting.Bone Rep.2018;9:47-60. [35] COLE LE, VARGO-GOGOLA T, ROEDER RK.Targeted delivery to bone and mineral deposits using bisphosphonate ligands.Adv Drug Deliv Rev. 2016;99(Pt A):12-27. [36] LAWSON MA, XIA Z, BARNETT BL, et al.Differences between bisphosphonates in binding affinities for hydroxyapatite.J Biomed Mater Res B Appl Biomater.2010;92(1):149-155. [37] NANCOLLAS GH, TANG R, PHIPPS RJ, et al.Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone.2006;38(5):617-627. [38] PULJULA E, TURHANEN P, VEPSALAINEN J, et al.Structural requirements for bisphosphonate binding on hydroxyapatite: NMR study of bisphosphonate partial esters.ACS Med Chem Lett. 2015;6(4):397-401. [39] BROWN JP, MORIN S, LESLIE W, et al.Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays.Can Fam Physician.2014;60(4):324-333. [40] CHOPRA I, ROBERTS M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev.2001;65(2):232-260. [41] NEALE JR, RICHTER NB, MERTEN KE, et al.Bone selective effect of an estradiol conjugate with a novel tetracycline-derived bone-targeting agent.Bioorg Med Chem Lett.2009;19(3):680-683. [42] CUTBIRTH ST.A Restorative Challenge: Tetracycline-Stained Teeth.Dent Today.2015;34(7):126,128-130. [43] ISHIZAKI J, WAKI Y, TAKAHASHI-NISHIOKA T,et al.Selective drug delivery to bone using acidic oligopeptides.J Bone Miner Metab. 2009;27(1):1-8. [44] JIANG T, YU X, CARBONE EJ, et al. Poly aspartic acid peptide-linked PLGA based nanoscale particles: potential for bone-targeting drug delivery applications.Int J Pharm.2014;475(1-2):547-557. [45] TAVAFOGHI M, CERRUTI M.The role of amino acids in hydroxyapatite mineralization.J R Soc Interface.2016;13(123).pii:20160462. [46] TAKAHASHI T, YOKOGAWA K, SAKURA N, et al.Bone-targeting of quinolones conjugated with an acidic oligopeptide.Pharm Res. 2008;25(12):2881-2888. [47] ZHANG Y, WEI L, MIRON RJ, et al. Anabolic bone formation via a site-specific bone-targeting delivery system by interfering with semaphorin 4D expression.J Bone Miner Res.2015;30(2):286-296. [48] ZHANG G, GUO B, WU H, et al.A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy.Nat Med. 2012;18(2):307-314. [49] WANG F, CHEN L, ZHANG R, et al.RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer.J Control Release.2014;196:222-233. [50] ADDISON WN, MILLER SJ, RAMASWAMY J, et al. Phosphorylation-dependent mineral-type specificity for apatite-binding peptide sequences. Biomaterials.2010;31(36):9422-9430. [51] DINJASKI N, PLOWRIGHT R, ZHOU S, et al.Osteoinductive recombinant silk fusion proteins for bone regeneration.Acta Biomater. 2017;49:127-139. [52] ZHU G, CHEN X.Aptamer-based targeted therapy.Adv Drug Deliv Rev. 2018;134:65-78. [53] CHENG H, CHAWLA A,YANG Y, et al. Development of nanomaterials for bone-targeted drug delivery. Drug Discov Today. 2017;22(9):1336-1350. [54] MIR M, AHMED N, REHMAN AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017; 159:217-231. [55] PERES C, MATOS AI, CONNIOT J, et al. Poly(lactic acid)-based particulate systems are promising tools for immune modulation. Acta Biomater. 2017;48:41-57. [56] XU Y, KIM CS, SAYLOR DM, et al.Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories.J Biomed Mater Res B Appl Biomater.2017;105(6):1692-1716. [57] MUSTAFA S, DEVI VK, PAI RS. Effect of PEG and water-soluble chitosan coating on moxifloxacin-loaded PLGA long-circulating nanoparticles. Drug Deliv Transl Res.2017;7(1):27-36. [58] ZHANG K, TANG X, ZHANG J, et al.PEG-PLGA copolymers: their structure and structure-influenced drug delivery applications.J Control Release. 2014;183:77-86. [59] CHEN X, CHEN J, LI B, et al.PLGA-PEG-PLGA triblock copolymeric micelles as oral drug delivery system: In vitro drug release and in vivo pharmacokinetics assessment.J Colloid Interface Sci.2017;490: 542-552. [60] INZANA JA, SCHWARZ EM, KATES SL, et al.Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials. 2016;81:58-71. [61] SEDGHIZADEH PP, SUN S, JUNKA AF, et al.Design, Synthesis, and Antimicrobial Evaluation of a Novel Bone-Targeting Bisphosphonate- Ciprofloxacin Conjugate for the Treatment of Osteomyelitis Biofilms. J Med Chem.2017;60(6):2326-2343. [62] LI CJ, LIU XZ, ZHANG L, et al.Advances in Bone-targeted Drug Delivery Systems for Neoadjuvant Chemotherapy for Osteosarcoma. Orthop Surg. 2016;8(2):105-110. [63] DE LA PUENTE P, AZAB AK. Contemporary drug therapies for multiple myeloma.Drugs Today (Barc).2013;49(9):563-573. [64] PATRIZI A, VENTURI M, DIKA E,e t al.Cutaneous adverse reactions linked to targeted anticancer therapies bortezomib and lenalidomide for multiple myeloma: new drugs, old side effects.Cutan Ocul Toxicol.2014;33(1):1-6. [65] ASAFO-ADJEI TA, CHEN AJ, NAJARZADEH A, et al.Advances in Controlled Drug Delivery for Treatment of Osteoporosis.Curr Osteoporos Rep. 2016;14(5):226-238. [66] LI CJ, CHENG P, LIANG MK, et al.MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation.J Clin Invest. 2015;125(4):1509-1522. |
| [1] | Chen Song, He Yuanli, Xie Wenjia, Zhong Linna, Wang Jian. Advantages of calcium phosphate nanoparticles for drug delivery in bone tissue engineering research and application [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3565-3570. |
| [2] | Gan Zhoujie, Pei Xibo. Enzyme-responsive nanoparticles in tumor therapy: superiority of nanoparticles in accumulation and drug release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2562-2568. |
| [3] | Li Xuan, Lu Min, Li Mingxing, Ao Meng, Tang Linmei, Zeng Zhen, Hu Jingwei, Huang Zhiqiang, Xuan Jiqing. In vitro multi-modal imaging of magnetic targeted nanoparticles and their targeting effect on hepatic stellate cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 566-571. |
| [4] | Gao Jianbo, Xia Bing, Li Shengyou, Yang Yujie, Ma Teng, Yu Peng, Luo Zhuojing, Huang Jinghui. Effect of nanoparticles carrying chondroitin sulfate ABC on the migration of Schwann cells in a magnetic field [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4526-4532. |
| [5] | Zhang Zhongyan, Li Yubo, Qi Tongning, Chang Tao. Three-dimensional printed polylactic acid resin humerus combined with bioactive coating promotes osteoblast adhesion and increases antibacterial ability [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(16): 2485-2492. |
| [6] | Li Ke, Xu Weiguo, Huang Chao, Zhang Zhiyu, Ding Jianxun. Polymer nanomedicines for osteosarcoma therapy [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(10): 1604-1614. |
| [7] | Zong Qiang, Xu Yanan, Qu Tianyi, Li Lijun, Hai Miti·Abuduaini, Ni Dongkui. Magnetic verapamil nanoparticles promote peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(34): 5425-5429. |
| [8] | Wang Yuan, Nie Fang, Wang Ting, Li Yan, Xu Ping. Systematic reviews of the accuracy of musculoskeletal ultrasound in the diagnosis of lateral epicondylalgia [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(32): 5233-5239. |
| [9] | Li Yongheng, Cui Yan, Zhang Zhiyu. Inhibitory effects of doxorubicin-loaded chitosan nanoparticles on osteosarcoma in mice [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(26): 4194-4199. |
| [10] | Du Jianhong, Fan Chunshui, Du Yuelian, Sun Xiaoyu, Wang Qingfeng. Preparation, sustained release and anti-tumor characteristics of hydroxypropyl chitosan/heparin nano-drug system [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(22): 3536-3541. |
| [11] | Cen Chaode, Zhang Yong, Luo Cong, Yang Xiaolan, Wu Jun, Wu Shengzhong, Liu Fuyao. Superparamagnetic chitosan gelatin microspheres as sustained-release gene carrier: magnetofection and release in vitro [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(2): 218-225. |
| [12] | Wu Jianxin, Luo Dan, Li Kun, Xu Ning, Ye Xiaojian. Preparation and property analysis of drug-loaded sustained-release scaffold with antibacterial and anti-inflammatory roles [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(18): 2858-2864. |
| [13] | Wang Runsheng, Liu Jianheng, Mao Keya, Tang Peifu. Material composite and properties of magnetic bone cement [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(18): 2921-2926. |
| [14] | Shen Mengjie, Yang Kun, Liu Qi . Application and research of iron oxide nanoparticles in bone tissue repair [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(14): 2248-2253. |
| [15] | Ma Jie, Wang Qian. Applications of functional Fe3O4 magnetic nanoparticles in biomedical field [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(10): 1626-1632. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||