Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (10): 1626-1632.doi: 10.3969/j.issn.2095-4344.1598
Previous Articles Next Articles
Applications of functional Fe3O4 magnetic nanoparticles in biomedical field
Ma Jie, Wang Qian
- Department of Orthopedics, Xijing Hospital of Air Force Medical University, Xi’an 710032, Shaanxi Province, China
-
Received:2018-11-26 -
Contact:Wang Qian, Associate chief nurse, Department of Orthopedics, Xijing Hospital of Air Force Medical University, Xi’an 710032, Shaanxi Province, China -
About author:Ma Jie, Nurse in charge, Department of Orthopedics, Xijing Hospital of Air Force Medical University, Xi’an 710032, Shaanxi Province, China
CLC Number:
Cite this article
Ma Jie, Wang Qian. Applications of functional Fe3O4 magnetic nanoparticles in biomedical field[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(10): 1626-1632.
share this article
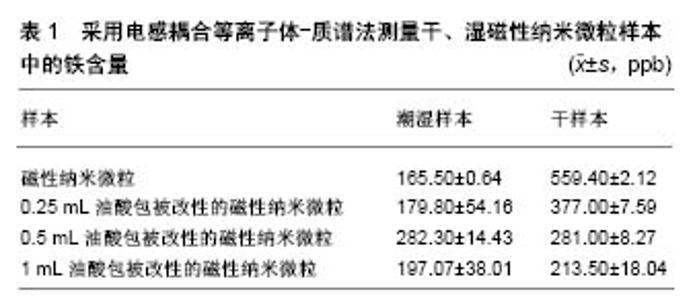
2.1 Fe3O4磁性纳米微粒的表面功能化 Fe3O4磁性纳米微粒的制备方法很多,主要包括沉淀法、溶胶-凝胶法、微乳液法、热分解法、水热法等。所制备的Fe3O4磁性纳米微粒容易发生氧化反应而聚集,表面缺乏偶联基团,限制了微粒的功能化,而且无法实现生物医学领域应用所要求的纳米材料高水溶性和高生物相容性。因此,要实现Fe3O4磁性纳米微粒的进一步应用,表面改性进行功能化处理非常重要。通过物理、化学的方法对Fe3O4磁性纳米微粒表面进行修饰、处理和加工,使纳米微粒的粒子结构、形貌、表面电荷状态、疏水性和应力性能等物理化学性质发生改变,使其更加稳定,减少互相聚集成团,避免粒子发生氧化反应,同时表面增加了活性功能基团,可携带药物及靶向配体,增强其生物相容性,减少内皮网状系统的吞噬及去除,增强其内吞效率。通常通过纳米微粒表面与改性材料之间进行化学吸附或化学反应的方法改变纳米微粒表面结构及性能,包括偶联剂法、酯化反应法、表面接枝靶向配体反应法等,聚合物分子、有机小分子及无机材料均可作为Fe3O4磁性纳米微粒的表面改性材料。 2.1.1 聚合物包覆Fe3O4磁性纳米微粒 聚合物包覆Fe3O4磁性纳米微粒对Fe3O4纳米微粒磁性的影响很小,同时聚合物制备时简单、可控性强。因此,聚合物是磁性纳米微粒表面改性材料的最重要组成部分[12-13]。聚合物可通过共价键、静电、配位等作用力结合到磁性纳米微粒表面,进而得到聚合物包覆的磁性纳米微粒;但此种方法也会随着反应的进行,显著增加空间位阻作用,已结合的聚合物不断遮蔽活性位点,致使接枝反应难以继续进行。聚合物的分子链越长反应结合率越低。另外,还可通过在磁性纳米微粒表面引入双键,在交联剂作用下与聚合物单体发生聚合反应,得到聚合物包覆的纳米粒子,因此避免了聚合物链遮蔽纳米粒子表面活性位点的不足。聚乙二醇、聚乙烯亚胺、壳聚糖、葡聚糖、淀粉等均是常用的磁性纳米微粒聚合物改性材料。在具体的实际应用中,可根据需求设计选取含有特定功能基团的聚合物而包覆及功能化Fe3O4磁性纳米微粒。 2.1.2 有机小分子改性Fe3O4磁性纳米微粒 硅烷偶联剂可与Fe3O4纳米微粒表面的羟基在有水环境下进行脱水反应,在无水环境下发生脱醇反应[14]。在离子液中,通过调节溶液的pH值使Fe3O4粒子与表面活性剂产生静电力,吸引获取有机小分子改性材料,表面活性剂结合到Fe3O4粒子表面后,疏水端向外,使粒子在溶液中具有良好的分散性,减少粒子之间的团聚[15]。Fe3O4纳米粒子表面含有大量铁原子,有机小分子可通过羧酸根、磷酸根等与这些未饱和配位的铁原子结合而嫁接于Fe3O4纳米微粒表面,Fe3O4纳米微粒与弱碱性的有机小分子水溶液按照一定比例混匀加热,即可完成Fe3O4纳米粒子的改性[16]。 2.1.3 无机材料包覆Fe3O4磁性纳米微粒 Fe3O4磁性纳米微粒与无机材料组成的壳-核结构,使Fe3O4磁性核心在无机材料构成的外壳保护下能够避免氧化、酸解而消磁,同时还能减少磁偶极子作用而降低团聚的发生,无机材料的外壳还能够作为进一步功能化的载体[17-19]。SiO2具有良好的生物相容性、耐酸及低毒性,被用来稳定Fe3O4磁性纳米微粒的性能。另外,SiO2表面拥有大量的羟基,为其进一步功能化提供了基础[20-21]。银等贵金属在生物检测、杀菌、细胞成像等领域发挥了重要作用,构建Fe3O4/贵金属纳米复合材料是非常有前瞻性的研究领域。Fe3O4/Ag纳米复合材料同时具有Fe3O4颗粒的超顺磁性、生物相容性及Ag颗粒的抗菌性能,在烧伤等学科拥有巨大应用潜力[22-24]。 2.2 超顺磁性Fe3O4纳米微粒在生物医学领域的进展 超顺磁性Fe3O4纳米微粒在生物医学领域特异性诊断及治疗方面均有应用,特有的超顺磁性及功能基团改性,使其在生物医学领域的应用潜力更为巨大。 2.2.1 靶向药物治疗 Wiidder等[25]率先提出了利用磁性纳米微粒作为载体进行靶向药物治疗的概念。将药物、生物活性物质等通过各种方式连接于磁性纳米粒子表面,以口服或者注射等途径到达体内,在足够大的外部梯度磁场作用下,结合有药物分子或其他活性分子的磁性纳米微粒复合物,聚集于病变部位而在局部发挥治疗作用,对正常机体无药物作用或仅有轻微作用,最大限度地减少了药物不良反应[26]。靶向给药在肿瘤治疗领域潜力巨大。超顺磁性Fe3O4纳米微粒作为载体搭载药物、生物活性物质,利用其超顺磁性特性还可控制病变部位的给药浓度,使药物富集于靶组织、靶器官,最大限度提高疗效,降低毒副作用[27]。赵明等[28]利用壳聚糖、胆固醇改性Fe3O4纳米微粒表面,结合紫杉醇后制备超顺磁性紫杉醇Fe3O4纳米微粒,该复合纳米微粒平均直径约20 nm,无明显团聚现象,磁滞曲线与Fe3O4磁流体类似,矫顽力与剩磁力几乎为零,复合超顺磁性特性;通过鼠尾静脉将超顺磁性紫杉醇Fe3O4纳米微粒注入体内,将头端放置于强度为0.5特斯拉的外部磁场中,磁靶向实验组大鼠脑组织紫杉醇浓度较单纯药物对照组提高5-30倍,局部药物浓度可维持>16 h,而且磁靶向实验组大鼠脑组织紫杉醇浓度在各观察时间点均高于非磁靶向组和单纯药物对照组。在磁靶向引导下,超顺磁性紫杉醇Fe3O4纳米微粒透过血脑屏障有效提高了脑组织中药物浓度,得以在靶器官发挥疗效。而且,超顺磁性Fe3O4纳米微粒不仅弥补了传统给药系统中药物无法到达特定病变位置、无法在病变局部选择性形成较高浓度而产生不良反应的缺陷,还为药物缓释发展提供了支持。有研究利用水热法制备了Fe3O4磁性纳米微粒,选用盐酸多西环素为模型药物,Fe3O4磁性纳米微粒在盐酸多西环素质量浓度0.1 g/L时,对药物的吸附率达到了46.3%,在不同pH值条件下均有不错的缓释性能;在pH=3时药物的缓释性能最佳,同时载药Fe3O4磁性纳米微粒具有良好的磁响应性及超顺磁性,为下一步靶向用药提供了可能。 2.2.2 核磁共振对比剂 核磁共振成像对软组织具有很高的分辨率,是临床上常用的诊断及病理研究手段,在临床应用中,通常需要借助对比剂增强或者减弱病变部位的信号,以便增大正常组织与病变组织之间的像差对比度。Fe3O4磁性纳米微粒以其超顺磁性特性在核磁共振成像中展现出了独特的对比剂功能,另外,因为良好的生物相容性、安全性及表面修饰改性能力,Fe3O4磁性纳米微粒已投入实际应用[29-34]。目前,已有多种氧化铁纳米微粒磁共振对比剂的商品化产品投入市场,如美国Advanced Magnetics 公司推出的基于氧化铁磁性纳米微粒的口服胃肠剂Ferumoxsil,1993年即在欧洲上市,1996年美国食品药品管理局又批准该公司推出肝脏血管对比剂Feridex ®(中文译名菲力磁®),还有淋巴对比剂也被批准上市。上述药物发挥治疗作用均是通过组织的摄取完成,是一种被动的靶向模式[35-37]。随着Fe3O4磁性纳米微粒制备工艺及表面修饰技术的更加成熟、完善,已获得具有主动功能、可用于恶性肿瘤鉴别诊断及早期诊断的全新Fe3O4磁性纳米微粒磁共振分子影像探针[38-39]。 Fe3O4磁性纳米微粒在临床早期的应用都是基于肝脏Kuffers细胞对氧化铁纳米颗粒的吞噬,在此基础上实现肝部成像。Combidex ®与Feridex ®均由右旋糖苷修饰磁性纳米颗粒而得,但Combidex ®粒径更小,在血液循环中停留时间更长。Weissleder等[30]利用产品Combidex ®在临床上突破性实现了为直径<2 mm的肿瘤淋巴结转移成像,具有非常重要的临床意义。近年来,随着纳米材料制备工艺不断进步及磁性纳米颗粒表面修饰改性技术的成熟,逐渐兴起了针对肿瘤早期诊断及鉴别诊断的分子成像技术研究热潮;该技术将超顺磁性Fe3O4纳米微粒与能够特异性识别肿瘤分子的生物活性分子偶联,构建了可主动识别肿瘤细胞的磁共振分子影像探针,进而实现了对肿瘤的早期诊断,显著提高肿瘤治愈率,必将成为肿瘤磁共振影像学诊断的重要进展方向。 2.2.3 肿瘤磁热疗 超顺磁性Fe3O4纳米微粒在功率足够大、频率足够高的交变磁场中,由于磁滞效应以及奈尔弛豫效应,将交变场能量转化为热量,使周围组织温度升高。肿瘤组织细胞比正常组织细胞对温度更为敏感,温度升到43-45 ℃时即会有大量肿瘤细胞发生凋亡,而正常细胞在此温度下可正常存活;利用这一细胞存活温度差及超顺磁性Fe3O4纳米微粒在交变磁场的产热效应,逐渐发展了肿瘤磁热疗技术。Gordon等[40]首先将这一治疗理念应用于抑制肿瘤,此后磁热疗技术抑制肿瘤逐渐引起了人们的关注。Jadhav等[41]利用油酸修饰超顺磁性Fe3O4纳米微粒表面,同时发现与未处理的Fe3O4纳米微粒比较,油酸处理的Fe3O4纳米微粒铁含量明显升高,表明随着油酸的包被,去掉了纳米微粒的水分,样本中铁的含量同样发生了变化,见表1。将改性的Fe3O4纳米微粒注射到小鼠纤维肉瘤细胞(WEHI-164)组织后,在交变磁场中进行磁热疗,在油酸改性超顺磁性Fe3O4纳米微粒后在其表面嫁接荧光探针,利用荧光发现超顺磁性Fe3O4纳米微粒主要在细胞膜发挥作用;利用锥虫蓝染色进一步发现,油酸改性处理的超顺磁性Fe3O4纳米微粒磁热疗肿瘤细胞后,细胞凋亡数量是未经油酸改性处理超顺磁性Fe3O4纳米微粒的5倍;因此,油酸改性处理的超顺磁性Fe3O4纳米微粒具有更强的肿瘤细胞高热杀伤能力,治疗效果明显。Jordan[42]制备出新的用于肿瘤治疗的纳米材料-纳米磁流体,进行肿瘤磁热疗,取得了重大进展。而且研究发现,相同温度的磁热疗法及热水处理肿瘤组织,磁热疗法能够引起更为严重的肿瘤细胞凋亡,磁热疗跟普通热疗相比拥有更强大的治疗作用[43]。 磁热疗不仅直接抑制肿瘤细胞,还能够诱导产生热休克蛋白,引发肿瘤免疫反应,进而杀伤肿瘤细胞,发挥治疗作用。Yanase等[44]在小鼠两侧均接种上胶质瘤细胞,只给右侧的肿瘤组织原位注射超顺磁性Fe3O4纳米微粒,然后暴露在交变磁场下,也即只有右侧肿瘤细胞接受了磁热疗,但结果显示两侧肿瘤细胞均被明显抑制。Ito等[45]进一步发现其中的机制,对侧肿瘤细胞被抑制是由于热诱导免疫杀伤肿瘤细胞而发挥治疗作用,他们发现这种对侧抑制现象在裸鼠身上并不能实现,只能在拥有完整免疫系统的正常小鼠身上观察到,这些研究结果也为治疗转移性肿瘤提供了新思路。 2.2.4 基因治疗 基因治疗中,基因的输送方式是非常关键的技术,传统的病毒载体往往有安全上的顾虑,非病毒载体可完全避免这一顾虑,近年来其开发研究得到了快速发展。磁转染技术的基础是目标或报道基因与磁性纳米微粒的集合,形成磁转染复合物;在体外基因转染中,将基因纳米微粒复合物加入到细胞培养皿中,在培养皿底部放置磁体或将其放置于电磁场中,加速纳米微粒的沉降,进而提高了转染速度,不同细胞系转染效率各有差异,但与脂质体对照组相比提高了5-10倍。在体内实验中,根据目标靶区位置在体表设置外部磁场,提高了基因转染速度,同时类似于靶向给药方式引导基因,聚集到体内的特定部位或器官。超顺磁性Fe3O4纳米微粒作为载体介导基因转染的技术,即磁转染,该技术利用超顺磁性Fe3O4纳米微粒搭载治疗或者报道基因,在外部梯度磁场的作用下,引导基因纳米微粒聚集到靶部位。外部磁场必需具有一定的磁场梯度,才能对其中的磁性Fe3O4纳米微粒产生磁性作用力,均匀磁场不能产生任何作用力,因此一般采用高梯度稀土元素磁体,实验条件下常用钕铁硼(NdFeB)磁体,但磁体磁力偏弱,甚至不能吸附距离体表仅数厘米的纳米颗粒,应用于人体显然无法达到目的。Schillinger等[46]成功利用磁转染技术输送SiRNA及反义寡聚核苷酸,并且发现该技术较其他非病毒载体极大地缩短了转染时间。Plank等[47]已证实磁转染技术转染水平不低于脂质体转染,而且已成功转染了呼吸道、血管组织细胞、肺上皮细胞[48]、角质形成细胞、软骨细胞、成骨细胞、血管内皮细胞[49]。与超顺磁性Fe3O4纳米微粒靶向给药类似,基因通常与表面经修饰改性的磁性纳米微粒结合[50-51]。磁转染技术加快了基因转染速度,并且大幅提高了转染率,同时作为基因载体超顺磁性Fe3O4纳米微粒的磁性特性,使基因的转送运输能够像靶向给药一样,在外部磁场引导下将其选择性运送至靶部位或靶器官,是非病毒载体基因转染技术中极具运用前景的技术之一。 2.2.5 磁性生物分离 超顺磁性Fe3O4纳米微粒的磁性载体技术与免疫学的结合,是其磁性生物分离的应用基础。将Fe3O4纳米微粒表面修饰改性使其功能化,偶联嫁接具有特异性结合特点的配体或受体,利用受体与配体的特异性结合,在外磁场作用下,可使标记的目标物质从组织、血液、体液中靶向快速分离。磁性生物分离技术同时兼备磁性与生物特性2大优势,可广泛应用于细胞、蛋白质、酶、核酸等物质的分离、纯化与检测[52]。Kuhara等[53]利用anti-hPCLP1单克隆抗体修饰超顺磁性Fe3O4纳米微粒,并利用其从人脐血中分离成血管细胞,分离的细胞中PCLP1阳性细胞纯度达到了95%。Xu等[54]将超顺磁性Fe3O4纳米微粒表面改性,利用其表面的的羧基偶联人表皮生长因子抗体(HER2),成功地从血液中分离出了乳腺癌细胞,为癌症的早期诊断提供了新策略。用于细胞分离的磁性纳米微球已有商品化产品面世,Stem cell公司研制的右旋糖苷磁性纳米微球和Miltenyi Biotech公司研制的多聚糖磁性纳米微球,均已被广泛应用于细胞的磁性生物分离。 "
| [1] 谭丽莎,孙明洋,胡运俊,等.功能化纳米Fe3O4磁性材料的制备及其对水中重金属离子的去除[J].化学进展, 2013,25(12): 2147-2158.[2] Sadat ME,Patel R,Sookoor J,et al. Effect of spatial confinement on magnetic hyperthermia via dipolar interactions in Fe3O4 nanoparticles for biomedical applications. Mater Sci Eng C Mater Biol Appl.2014;42:52-63.[3] Kiani M,Hendijani M,Mohammadipour M,et al. Design, Preparation and Characterization of MoO3H-functionalized Fe3O4@SiO2 Magnetic Nanocatalyst and Application for the One-pot Multicomponent Reactions.Acta Chim Slov. 2017; 64(3):707-713.[4] 李黎,马力.Fe3O4磁性微粒的制备及表征[J].中国组织工程研究与临床康复,2011,15(34): 6385-6387.[5] Yazdani F,Fattahi B,Azizi N.Synthesis of functionalized magnetite nanoparticles to use as liver targeting MRI contrast agent. J Magn Magn Mater.2016;406:207-211.[6] 李瑶,崔海信,刘琪,等.磁性纳米颗粒作为基因载体的研究发展概况[J].功能材料,2010,41(S1):14-19.[7] 刘坤,陈良勇,蒋恒,等. Fe3O4磁性纳米微粒的制备及药物缓释性能的研究[J].广州化工,2014,42(4):66-68. [8] Liu X,Li L,Liu YQ,et al. Ultrasensitive detection of deltamethrin by immune magnetic nanoparticles separation coupled with surface plasmon resonance sensor.Biosens Bioelectron. 2014;59:328-334.[9] 李静,秦晓民,丁焕平,等.磁性纳米微粒分离中药蛋白质的实验研究[J].生物磁学,2005,5(2):1-4.[10] Yuan B,Yang XQ,Xue LW,et al. A novel recycling system for nano-magnetic molecular imprinting immobilised cellulases: Synergistic recovery of anthocyanin from fruit and vegetable waste.Bioresour Technol.2016;222:14-23.[11] Rabban M,Rafiee F,Ghafuri H.Synthesis of Fe3O4 nonoparticles via a fast and facile machanochemical method: modification of surface with porphyrin and photocatalytic study. Mater Lett.2016;166:247-250.[12] Rezayan AH, Mosavi M,Kheirjou S,et al.Monodisperse magnetite (Fe3O4) nanoparticles modified with water soluble polymers for the diagnosis of breast cancer by MRI method.J Magn Magn Mater.2016;420:210-217.[13] Yu L, Hao G,Gu J,et al. Fe3O4/PS magnetic nanoparticles: Synthesis, characterization and their application as sorbents of oil from waste water. J Magn Magn Mater. 2015;394(15): 14-21.[14] Shaterian HR,Aghakhanizadeh M. Minopropyl coated on magnetic Fe3O4 and SBA-15 nanoparticles catalyzed mild preparation of chromeno[2, 3-d]pyrimidines under ambient and solvent-free conditions.Catalys Sci Technol. 2013;3(2): 425-428.[15] Chen L,Wang T,Tong J.Application of derivatized magnetic materials to the separation and the preconcentration of pollutants in water samples. TrAC Trend Anal Chem. 2011; 30(7):1095-1108.[16] Gao Q,Feng Y.Magnetic micro-/nano-materials: functionalization and their applications in pretreatment for food samples.Se Pu.2014;32(10):1043-1051.[17] Farmany A,Mortazavi SS,Mahdavi H,et al.Ultrasond-assisted synthesis of Fe3O4/SiO2 core/shell with enhanced adsorption capacity for diazinon removal.J Magn Magn Mater. 2016; 416:75-80.[18] Fang W,Zheng J,Chen C,et al.One-pot synthesis of porous Fe3O4 shell/silver core nanocomposites used as recyclable magnetic antibacterial agents J Magn Magn Mater. 2014; 357:1-6. [19] Qiao M,Lei X,Ma Y,et al.Facile synthesis and enhanced electromagnetic microwave absorption performance for porous core-shell Fe3O4@MnO2 composite microspheres with lightweight feature. J Alloys Compd.2017;693:432-439.[20] Bagwe RP,Hilliard LR,Tan W.Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir.2006;22(9):4357-4362.[21] Peng X,Liu WS,Shi HC.Functionalized magnetic core–shell Fe3O4@SiO2 nanoparticles as selectivity-enhanced chemosensor for Hg(II).Dyes Pigments.2011;91(1):26-32.[22] 李亚斋,王虹. Fe3O4-Ag 磁性复合纳米颗粒的制备及其生物医学应用研究进展[J].化工新型材料, 2015,43(4):229-231.[23] Du JJ,Jing CY.Preparation of Thiol Modified Fe3O4@Ag Magnetic SERS Probe for PAHs Detection and Identification.J Phys Chem C.2011;115(36):17829-17835.[24] Chudasama B,Vala AK,Andhariya N,et al.Enhanced antibacterial activity of bifunctional Fe3O4-Ag core-shell nanostructures. Nano Res.2009;2(12):955-965.[25] Widder KJ,Senyel AE,Scarpelli GD.Magnetic microspheres: a model system of site specific drug delivery in vivo.Proc Soc Exp Biol Med.1978;158(2):141-146.[26] Lubbe AS,Alexiou C,Bergemann C.Clinical applications of magnetic drug targeting.J Surg Res.2001;95(2):200-206.[27] Kubo T,Sugita T,Shimose S,et al.Targeted delivery of anticancer drugs with intravenously administered magnetic liposomes in osteosarcoma-bearing hamsters.Int J Oncol. 2000;17(2):309-315.[28] 赵明,梁超,李安民,等.超顺磁性紫杉醇纳米微粒对中枢神经系统肿瘤磁靶向药物化疗的实验研究[J].中国现代神经疾病杂志, 2009,9(6):589-592.[29] Degen CL,Poggio M,Mamin HJ,et al. Nanoscale magnetic resonance imaging.Proc Natl Acad Sci U S A. 2009;106(5): 1313-1317.[30] Weissleder R,Pittet MJ.Imaging in the era of molecular oncology. Nature.2008;452(7187): 580-589.[31] Sun C,Lee JS,Zhang M. Magnetic nanoparticles in MR imaging and drug delivery.Adv Drug Deliv Rev. 2008;60(11): 1252-1265.[32] Jun YW,Lee JH,Cheon J.Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew Chem Int Ed Engl.2008;47(28):5122-5135.[33] Corot C,Robert P,Idée JM,et al.Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev.2006;58(14):1471-1504.[34] Qiao R,Yang C,Gao M.Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications.J Mater Chem.2009;19(35):6274.[35] Hu FQ,Chen J,Qi Y,et al.Preparation of Biocompatible Magnetite Nanocrystals for In Vivo Magnetic Resonance Detection of Cancer.Adv Mater.2006;18(19):2553-2556.[36] Lee JH,Huh YM,Jun YW,et al.Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging.Nat Med. 2007;13(1):95-99.[37] Harisinghani MG,Barentsz J,Hahn PF,et al.Noninvasive detection of clinically occult lymph-node metastases in prostate cancer.N Engl J Med.2003;348(25):2491-2199.[38] Huh YM,Jun YW,Song HT,et al.In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals.J Am Chem Soc.2005;127(35):12387-12391.[39] Veiseh O,Sun C,Fang C,et al.Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier.Cancer Res. 2009; 69(15):6200-6207.[40] Gordon RT,Hines JR,Gordon D.Intracellular hyperthermia. A biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med Hypotheses. 1979;5(1):83-102.[41] Jadhav NV,Prasad AI,Kumar A, et al. Synthesis of oleic acid functionalized Fe3O4 magnetic nanoparticles and studying their interaction with tumor cells for potential hyperthermia applications.Colloids Surf B Biointerfaces. 2013;108:158-168.[42] Jordan A. Hyperthermia classic commentary: 'Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia' by Andreas Jordan et al.,International Journal of Hyperthermia, 1993;9:51-68.Int J Hyperthermia. 2009;25(7):512-516.[43] Rodríguez-Luccioni HL,Latorre-Esteves M,Méndez-Vega J,et al.Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles.Int J Nanomedicine. 2011; 6:373-380.[44] Yanase M,Shinkai M,Honda H,et al.Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes.Jpn J Cancer Res.1998;89(7):775-782.[45] to A,Shinkai M,Honda H, et al.Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia.Cancer Immunol Immunother. 2001;50(10): 515-522.[46] Schillinger U,Brilla T,Rudolphb C,et al.Advances in magnetofection—magnetically guided nucleic acid delivery. J Magn Magn Mater.2005;293(1):501-508.[47] Plank C,Schillinger U,Scherer F,et al.The magnetofection method: using magnetic force to enhance gene delivery.Biol Chem.2003;384(5):737-747.[48] Gersting SW,Schillinger U,Lausier J,et al.Gene delivery to respiratory epithelial cells by magnetofection. J Gene Med. 2004;6(8):913-922.[49] Krötz F,Sohn HY,Gloe T,et al.Magnetofection potentiates gene delivery to cultured endothelial cells. J Vasc Res. 2003;40(5): 425-434.[50] Neuberger T,Schöpfa B,Hofmannb H,et al. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. 2005;293(1):483-496.[51] Harris LA,Goff JD,Carmichael AY,et al.Magnetite Nanoparticle Dispersions Stabilized with Triblock Copolymers.Chem Mater. 2003;15(6):1367-1377.[52] Dong X,Zheng Y,Huang Y,et al.Synthesis and characterization of multifunctional poly(glycidyl methacrylate) microspheres and their use in cell separation.Anal Biochem. 2010;405(2): 207-212.[53] Kuhara M,Yoshino T,Shiokawa M,et al.Magnetic separation of human podocalyxin-like protein 1 (hPCLP1)-positive cells from peripheral blood and umbilical cord blood using anti-hPCLP1 monoclonal antibody and protein A expressed on bacterial magnetic particles.Cell Struct Funct. 2009;34(1): 23-30.[54] Xu H,Aguilar ZP,Yang L,et al.Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood.Biomaterials.2011;32(36):9758-9765.[55] Bigham A,Hassanzadeh-Tabrizi SA,Rafienia M,et al.Ordered mesoporous magnesium silicate with uniform nanochannels as a drug delivery system: The effect of calcination temperature on drug delivery rate.Ceram Int. 2016;42: 17185-17191.[56] Hassanzadeh-Tabrizi SA,Bigham A,Rafienia M. Surfactant- assisted sol-gel synthesis of forsterite nanoparticles as a novel drug delivery system.Mat Sci Eng C Bio.2016;58: 737-741.[57] Hadidi M,Bigham A,Saebnoori E,et al. Electrophoretic- deposited hydroxyapatite-copper nanocomposite as an antibacterial coating for biomedical applications.Surf Coat Technol. 2017;321:171-179.[58] Liu J,Sun Z,Deng Y,et al.Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups.Angew Chem Int Ed Engl. 2009; 48(32):5875-5879. [59] Bogart LK,Pourroy G,Murphy CJ,et al.Nanoparticles for imaging, sensing, and therapeutic intervention.ACS Nano. 2014;8(4):3107-3122.[60] Ghadiri S,Hassanzadeh-Tabrizi SA,Bigham A.The effect of synthesis medium on structure and drug delivery behavior of CTAB-assisted sol–gel derived nanoporous calcium- magnesium-silicate.J Sol Gel Sci Technol. 2017;83: 229-236.[61] Khamsehashari N,Hassanzadeh-Tabrizi SA,Bigham A.Effects of strontium adding on the drug delivery behavior of silica nanoparticles synthesized by P123-assisted sol-gel method. Mat Chem Phys.2018;205:283-291.[62] Kim S,Katsumata K,Okada K,et al.Porous magnetite secondary particles prepared by surfactant-free solvothermal method with non-contact heat-assisted drug releasing property. Adv Powder Technol.2016;27:513-520. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||