Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (2): 218-225.doi: 10.3969/j.issn.2095-4344.1510
Previous Articles Next Articles
Superparamagnetic chitosan gelatin microspheres as sustained-release gene carrier: magnetofection and release in vitro
Cen Chaode1, Zhang Yong2, Luo Cong3, Yang Xiaolan4, Wu Jun3, Wu Shengzhong1, Liu Fuyao1
-
Received:2018-07-21Online:2019-01-18Published:2019-01-18 -
Contact:Luo Cong, Chief physician, Department of Orthopaedics, Children’s Hospital of Chongqing Medical University, Chongqing 400014, China -
About author:Cen Chaode, Master, Physician, Department of Orthopaedics, Guizhou Provincial Orthopedics Hospital, Guiyang 550000, Guizhou Province, China -
Supported by:Chongqing Science and Technology Research Project, No. CSTC2011ggB1004 (to LC); the National Clinical Key Specialty Construction Project, No. [2013]544
CLC Number:
Cite this article
Cen Chaode, Zhang Yong, Luo Cong, Yang Xiaolan, Wu Jun, Wu Shengzhong, Liu Fuyao. Superparamagnetic chitosan gelatin microspheres as sustained-release gene carrier: magnetofection and release in vitro[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(2): 218-225.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
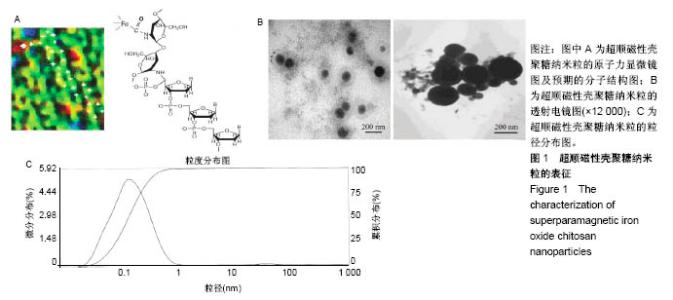
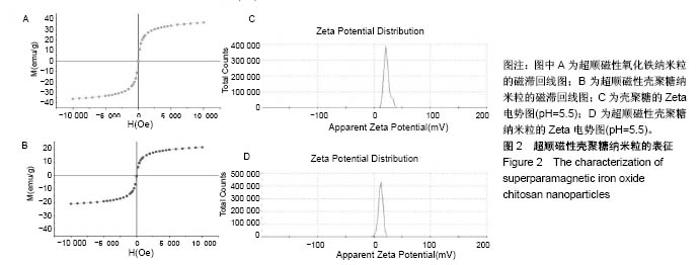
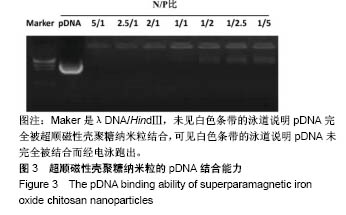
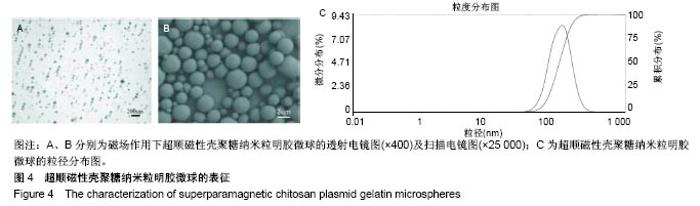
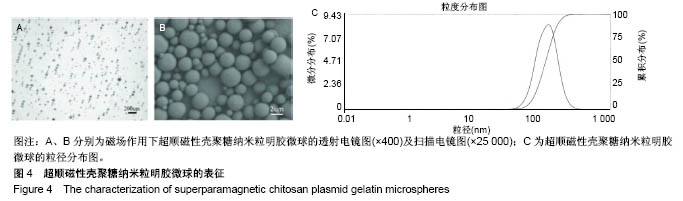
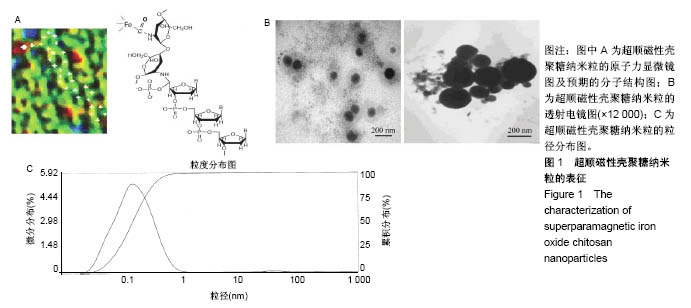
2.1 SPIOCN的表征 图1A为原子力显微镜下SPIOCN/ pDNA复合物的彩阶形貌图,亮区为高处,暗区为低处,链上红点为Fe3O4基团的所在位置,Fe原子通过酰胺键与壳聚糖连接。质粒DNA经带负电荷的磷酸基团与壳聚糖带正电荷的NH-基团静电吸附,DNA五元环之间由磷原子连接,与图1A右侧预期的分子结构图是相符合的。 图1B所示透射电镜下SPIOCN的外形呈圆形或椭圆形,均匀度尚可,粒径约200 nm。图1C所示为SPIOCN的粒径分布图,平均粒径(187±24) nm;粒径累计百分数:10%为58 nm,50%为150 nm,90%为366 nm,97%为 524 nm,65.37%≤200 nm,83.58%≤300 nm,径距= 2.53;曲线拟合系数为0.80。 图2A显示:随着外加磁场强度的增大,样品的磁化强度值相应增大,超顺磁性氧化铁纳米粒的饱和磁化强度为(36±3) emu/g。当外加磁场为0 Oe时,剩磁为0.0174 emu/g (接近零),提示磁响应性好,具有超顺磁性。图2B显示:超顺磁性氧化铁纳米粒经与壳聚糖反应形成SPIOCN后饱和磁化强度有所降低,为(20.3±4.5) emu/g,但仍保持良好的超顺磁性。 图2C所示壳聚糖的Zeta电位为(21.6±2.5) mV,由于超顺磁性氧化铁纳米粒表面的羧基中和部分正电荷,使得壳聚糖与超顺磁性氧化铁纳米粒反应形成的SPIOCN电位下降,为(9.5±2.4) mV。SPIOCN虽然电位降低但依然带正电荷,易于与带负电的质粒DNA通过静电吸附结合。"
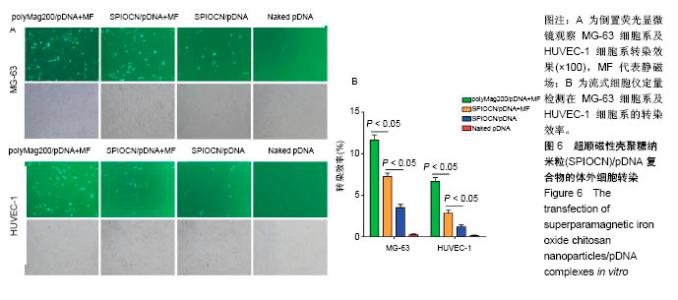
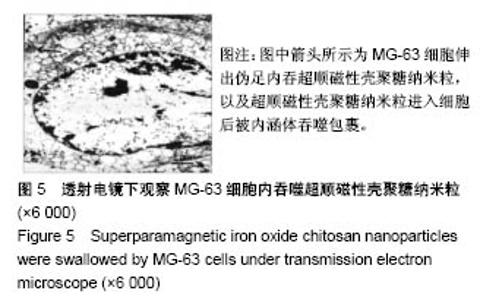
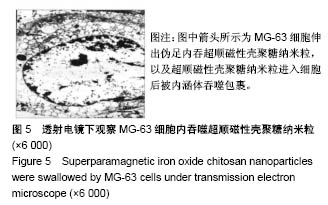
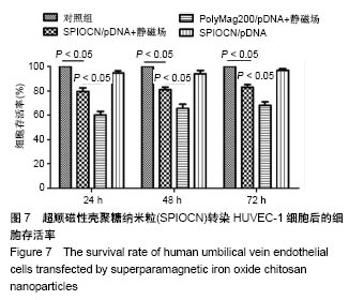
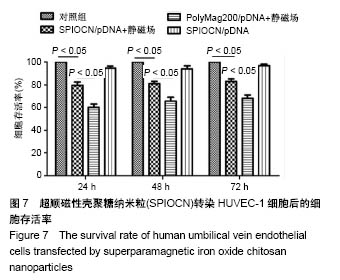
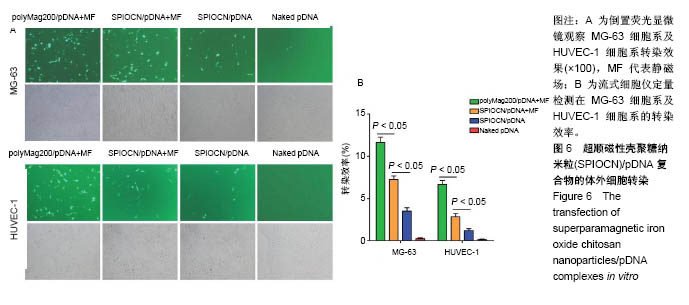
2.5 SPIOCN/pDNA的体外细胞转染 转染24 h后倒置荧光显微镜下观察可见,PolyMag200/pDNA+静磁场组转染效率最高,见图6A,而SPIOCN/pDNA+静磁场组绿色荧光表达量及强度明显高于SPIOCN/pDNA组,表明SPIOCN能结合保护质粒不被溶酶体降解并完成了溶酶体逃逸而实现转染,静磁场能显著提高SPIOCN/pDNA的转染率。裸质粒转染率低下的原因主要在于被当被细胞内吞后形成内涵体,内涵体被溶酶体吞噬时质粒大量降解。流式细胞仪定量测定细胞转染率,见图6B,在MG-63细胞中,PolyMag200/pDNA+静磁场组转染率为(11.6±2.4)%,SPIOCN/pDNA+静磁场组转染率为(7.2±1.5)%,SPIOCN/ pDNA组转染率为(3.5±0.7)%,裸pDNA组转染率为(0.2±0.1)%,组间比较差异有显著性意义(P < 0.05)。在HUVEC-1细胞中,PolyMag200/pDNA+静磁场组转染率为(6.7±1.4)%,SPIOCN/pDNA+静磁场组转染率为(2.8±0.6)%,SPIOCN/pDNA组转染率为(1.2±0.4)%,裸pDNA组转染率为(0.1±0.1)%,组间比较差异有显著性意义(P < 0.05)。"
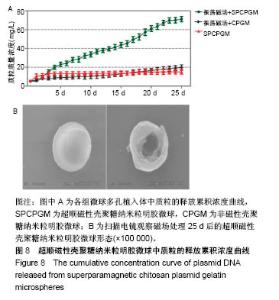
2.7 载SPCPGM骨支架中pDNA的体外释放 多孔骨植入体中SPCPGM及非磁性壳聚糖质粒明胶微球在振荡磁场下质粒释放的累积浓度曲线,见图8A。从中可见各组微球体外质粒溶出时间均超过3周,有明显缓释作用。A组从第1天即开始加用振荡磁场,每日药物溶出量明显高于另两组(4倍左右)。统计学方差分析,A组与B、C组之间药物溶出量均有显著性差异(q检验,P < 0.001);B组与C组药物溶出量比较差异无显著性意义(q检验,P > 0.05)。在实验的第22-25天,振荡磁场+SPCPGM组溶出速率减慢,此时质粒的释药百分率接近70%。而未用振荡磁场SPCPGM组质粒的释药百分率约为11%,壳聚糖质粒明胶微球+磁场组约为15%。可以认为,SPCPGM及非磁性壳聚糖质粒明胶微球多孔植入体中质粒缓释时间还可持续较长时间。在实验条件下,振荡磁场能促进含SPCPGM多孔骨植入体中质粒溶出,3周时能增加原有的质粒溶出量达4倍。 SPCPGM释放前为完整的球形,释放实验后扫描电镜观察显示SPCPGM有空洞形成,见图8B所示,提示SPIOCN包裹于明胶分子内或与明胶分子相嵌,超顺磁性微球整体随振荡磁场运动,起到了局部搅拌器的作用。磁性纳米Fe3O4在微球中振动,使微球产生了缝隙。另外,加上纳米粒振动的挤压和吸引作用,建立了药物渗透通道。"

| [1] Duan X,Li W,Xiang Z.Research progress of angiogenesis in vascularized tissue engineered bone.Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.2015,29(2):239-244. [2] Nakano K,Murata K,Omokawa S,et al.Promotion of Osteogenesis and Angiogenesis in Vascularized Tissue-Engineered Bone Using Osteogenic Matrix Cell Sheets.Plast Reconstr Surg. 2016;137(5): 1476-1484.[3] Chen K,Zhang C,Wang L,et al.Progress on strategies to promote vascularization in bone tissue engineering.Zhongguo Gu Shang. 2015;28(4):383-388.[4] Davies N,Dobner S,Bezuidenhout D,et al.The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials.2008;29(26):3531-3538.[5] CasselI OC,Hofer SO,Morrison WA,et al.Vascularisation of tissue-engineered grafts:the regulation of angiogenesis in reconstructive surgery and in disease states.Br J Plast Surg. 2002;55(8):603-610.[6] 金丹,陈滨,裴国献,等.筋膜瓣促组织工程骨再血管化及山羊长段骨缺损的修复[J].中华实验外科杂志,2005,22(3):269-271. [7] Jayaraman P,Gandhimathi C,Venugopal JR,et al.Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering.Adv Drug Deliv Rev.2015,94:77-95.[8] Yin J,Qiu S,Shi B,et al.Controlled release of FGF-2 and BMP-2 in tissue engineered periosteum promotes bone repair in rats. Biomed Mater.2018;13(2):025001.[9] Witlox MA,Lamfers ML,Wuisman PI,et al.Evolving gene therapy approaches for osteosarcoma using viral vectors: review. Bone. 2007;40(4):797-812.[10] Hartono SB,Yu M,Gu W,et al.Synthesis of multi-functional large pore mesoporous silica nanoparticles as gene carriers. Nanotechnology.2014;25(5):055701.[11] Wang Y,Li L,Shao N,et al.Triazine-modified dendrimer for efficient TRAIL gene therapy in osteosarcoma.Acta Biomater. 2015;17: 115-124.[12] Wu P,Chen H,Jin R,et al.Non-viral gene delivery systems for tissue repair and regeneration.J Transl Med.2018;16(1):29.[13] Helal NA,Osami A,Helmy A,et al.Non-viral gene delivery systems: hurdles for bench-to-bedside transformation. Pharmazie. 2017; 72(11):627-693.[14] Ishii T,Okahata Y,Sato T.Mechanism of cell transfection with plasmid/chitosan complexes. Biochim Biophys Acta. 2001; 1514(1):51-64.[15] Vainauska D,Kozireva S,Karpovs A,et al.A novel approach for nucleic acid delivery into cancer cells.Medicina(Kaunas). 2012; 48(6):324-329.[16] Smolders S,Kessels S,Smolders SM,et al.Magnetofection is superior to other chemical transfection methods in a microglial cell line.J Neurosci Methods.2017;293:169-173.[17] Laurentt N,Sapet C,Le GL,et al.Nucleic acid delivery using magnetic nanoparticles: the Magnetofection technology.Ther Deliv.2011;2(4):471-482.[18] Crane GM,Ishaug SL,Mikos AG.Bone tissue engineering.Nat Med.1995;1(12):1322-1324.[19] Carmeliet P.Mechanisms of angiogenesis and arteriogenesis.Nat Med.2000,6(4):389-395.[20] Oda M.Prefabrication of vascularized bone grafts measured with laser Doppler flowmetry: An experimental study in rats.Scand I Plast Reconstr Surg Hand Surg. 1994;28:249-252.[21] Wamke PH,Springer IN,Wiltfang J.Growth and transphantation of a custom vascularised bone graft in a man. Lancet. 2004;364 (9436):766-770. [22] 汪群力,裴国献.组织工程组织血管化研究新进展[J].中华创伤骨科杂志,2005,7(6):564-567.[23] Kasper FK,Melville J,Shum J,et al.Tissue Engineered Prevascularized Bone and Soft Tissue Flaps.Oral Maxillofac Surg Clin North Am.2017;29(1):63-73.[24] Kaempfen A,Todorov A,Güven S,et al.Engraftment of Prevascularized, Tissue Engineered Constructs in a Novel Rabbit Segmental Bone Defect Model.Int J Mol Sci. 2015;16(6): 12616-12630.[25] Nguyen LH,Annabi N,Nikkhah M,et al.Vascularized Bone Tissue Engineering:Approaches for Potential Improvement.Tissue Eng Part B Rev.2012;18(5):363-382.[26] Bussemer T,Dashevsky A,Bodmeier R.A pulsatile drug delivery system based on rupturable coated hard gelatin capsules.J Control Release.2003;93(3):331-933.[27] Solorio L,Zwolinski C,Lund AW,et a1.Gelatin microspheres crosslinked with genipin for local delivery of growth factors.J Tissue Eng Regen Med.2010;4(7):514-523.[28] Jabbarzadeh E,Starnes T,Khan YM,et al.Induction of angiogenesis in tissue-engineered scafolds designed for bone repair:A combined gene therapy-cell transplantation approach.Proc NatI Acad Sci USA. 2008;105(32):11099-11104.[29] Yang W,Wang F,Feng L,et al.Applications and prospects of non-viral vectors in bone regeneration.Curr Gene Ther.2018.doi:10.2174/1566523218666180227154232.[30] Hardee CL,Arévalo-Soliz LM,Hornstein BD,et al.Advances in Non-Viral DNA Vectors for Gene Therapy. Genes(Basel). 2017;8(2).pii:E65.doi:10.3390/genes8020065.[31] Slivac I,Guay D,Mangion M,et al.Non-viral nucleic acid delivery methods.Expert Opin Biol Ther. 2017;17(1):105-118.[32] Long Z,Zhang J,Shen Y,et al.Polyethyleneimine grafted short halloysite nanotubes for gene delivery.Mater Sci Eng C Mater Biol Appl.2017;81:224-235.[33] Del Pozo-Rodríguez A,Solinís MÁ,Rodríguez-Gascón A,et al. Applications of lipid nanoparticles in gene therapy.Eur J Pharm Biopharm.2016;109:184-193.[34] Raftery R,O'Brien FJ,Cryan SA.Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules. 2013; 18(5):5611-5647.[35] Sohrabijam Z,Saeidifar M,Zamanian A,et al.Enhancement of magnetofection efficiency using chitosan coated superparamagnetic iron oxide nanoparticles and calf thymus DNA.Colloids Surf B Biointerfaces.2017;152:169-175.[36] Pan Z,Shi Z,Wei H,et al.Magnetofection based on superparamagnetic iron oxide nanoparticles weakens glioma stem cell proliferation and invasion by mediating high expression of microRNA-374a. J Cancer. 2016;7(11):1487-1496.[37] Wang YX,Xuan S,Port M,et al.Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research.Curr Pharm Des. 2013;19(37): 6575-6593.[38] Wang X,Chen B,Yang X,et al.Functionalized superparamagnetic nanoparticles for highly-efficient gene delivery.J Nanosci Nanotechnol.2013;13(2):746-750.[39] Moghimi SM,Symonds P,Murray JC,et al.A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy.Mol Ther.2005;11(6):990-995. [40] Edelman ER,Kost J,Bobeck H,et al.Regul- ation of drug release from polymer matrices by oscillating magnetic fields.J Biomed Mater Res.1985;19:67-83. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Li Li, Ma Li. Immobilization of lactase on magnetic chitosan microspheres and its effect on enzymatic properties [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 576-581. |
| [6] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [7] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [8] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [9] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [10] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [11] | Li Jun, Zuo Xinhui, Liu Xiaoyuan, Zhang Kai, Han Xiangzhen, He Huiyu, . Effect of over expression of miR-378a on osteogenic and vascular differentiation of bone marrow mesenchymal stem cell sheet [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 4939-4944. |
| [12] | Li Ye, Yang Yukun, Zhu Xiangqing, He Jie, Wang Jinxiang, Wang Yanying, Tian Chuan, Pang Rongqing, Pan Xinghua. In vivo track technique for mesenchymal stem cells: how to realize simultaneous tracing of distribution and survival [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 5025-5033. |
| [13] | Shi Qin, Sun Baolan, Yang Xiaoqing, Zhang Yuquan . Mesenchymal stem cell-derived exosomes carrying miRNAs in tissue repair and treatment of related diseases: application and advantages [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 5040-5045. |
| [14] | Wang Xinwei, Zhao Yingjie, Chang Yan, Wei Wei. Mesenchymal stem cells in the treatment of cartilage damage in osteoarthritis: role, application, and problem [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 5053-5058. |
| [15] | Liu Xiaogang, Li Tian, Zhang Duo. Research hotspot of seed cell selection in cartilage tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 5059-5064. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||