Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (10): 1604-1614.doi: 10.3969/j.issn.2095-4344.2221
Previous Articles Next Articles
Polymer nanomedicines for osteosarcoma therapy
Li Ke1, 2, Xu Weiguo2, Huang Chao2, 3, Zhang Zhiyu1, Ding Jianxun2
- 1Department of Orthopedics, The Fourth Affiliated Hospital of China Medical University, Shenyang 110032, Liaoning Province, China; 2Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin Province, China; 3Department of Hand Surgery, The Second Hospital of Jilin University, Changchun 130041, Jilin Province, China
-
Received:2019-05-25Revised:2019-06-13Accepted:2019-07-20Online:2020-04-08Published:2020-02-18 -
Contact:Zhang Zhiyu, MD, Professor, Department of Orthopedics, The Fourth Affiliated Hospital of China Medical University, Shenyang 110032, Liaoning Province, China -
About author:Li Ke, Master candidate, Department of Orthopedics, The Fourth Affiliated Hospital of China Medical University, Shenyang 110032, Liaoning Province, China; Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin Province, China -
Supported by:the National Natural Science Foundation of China, No. 51873207, No. 51673190
CLC Number:
Cite this article
Li Ke, Xu Weiguo, Huang Chao, Zhang Zhiyu, Ding Jianxun. Polymer nanomedicines for osteosarcoma therapy[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(10): 1604-1614.
share this article
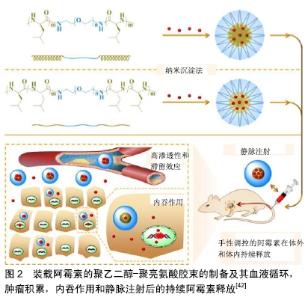
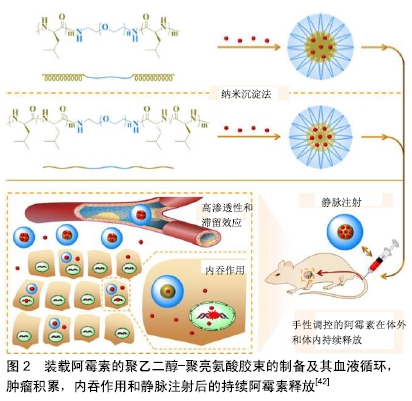
2.1 小分子药 2.1.1 阿霉素 阿霉素是一种广谱抗肿瘤药,具有强烈的细胞毒性,其作用机制主要是嵌入DNA而抑制核酸的合成。由于阿霉素的毒性和快速血浆清除率,预防正常脏器损伤和延长药物血液循环时间已成为现在的研究重点。与传统的阿霉素治疗相比,阿霉素负载于纳米粒子系统,可提高药物的抗肿瘤性能。胶束是在水性介质中两亲性嵌段共聚物的自组装而成的纳米级核壳结构,其结构仅在共聚物的临界胶束浓度下形成。Arg-Gly-Asp (RGD)是一种亲细胞肽,能够与αvβ3和αvβ5整合素相互作用,并且广泛表达于MG-63,MNNG/HOS等骨肉瘤细胞系[40]。FANG等[41]设计并开发了一种新型阿霉素负载的靶向高分子胶束(RGD-PEG?PM),由RGD封端的聚乙二醇-聚三亚甲基碳酸酯(poly(ethylene glycol)-poly (trimethylene carbonate),PEG?PM)两亲性可生物降解嵌段共聚物自组装形成,用于骨肉瘤的高效靶向化疗。在生理条件下,来自RGD-阿霉素-PM的阿霉素在60 h内释放达到63%。通过MTT检测,RGD-阿霉素-PM对MG-63骨肉瘤细胞的半抑制浓度远低于其非靶向治疗组(阿霉素-PM),这意味着RGD修饰纳米粒子增强了细胞靶向能力,并导致更有效的抗肿瘤作用。此外,通过体外流式细胞术和共聚焦激光扫描显微镜成像分析证实了RGD-阿霉素-PM的靶向能力。因此,这种RGD修饰的载阿霉素高分子胶束有望用于骨肉瘤的靶向化疗。DING等[42]的团队以L-亮氨酸N-内羧酸酐为引发剂,或等效的D-亮氨酸N-内羧酸酐和L-亮氨酸N-内羧酸酐为引发剂,通过开环聚合合成了2种三嵌段聚乙二醇-聚亮氨酸共聚物。两亲共聚物在水性环境中自组装成球形胶束聚集体,然后通过纳米沉淀将阿霉素加载到胶束中。研究发现聚乙二醇-聚D,L-亮氨酸胶束显示出更高的载药效力,并且装载阿霉素的聚乙二醇-聚D,L-亮氨酸胶束释放速率较慢。更重要的是,装载阿霉素的胶束,尤其是具有聚D,L-亮氨酸核心的胶束,在体内显示出增强的肿瘤抑制力和安全性[42]。因此,装载阿霉素的聚乙二醇-聚亮氨酸胶束似乎有希望用于骨肉瘤的治疗,见图2。因此,多肽胶束在体内控制递送阿霉素用于骨肉瘤的治疗具有光明的前景。 "
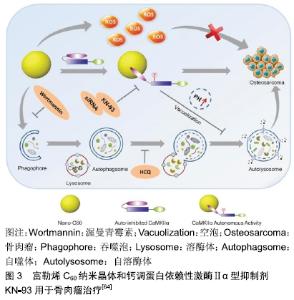
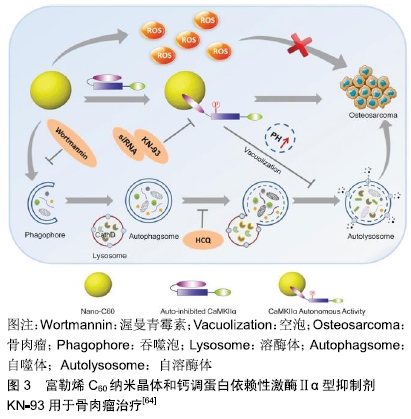
脂质体是一种封闭的双层囊泡,具有良好的生物相容性,已成功用于各种抗肿瘤药物的输送。脂质体通常用合适的高分子如聚乙二醇进行改性,可以改善结构稳定性并延长循环半衰期[43-44]。HAGHIRALSADAT等[45]通过pH梯度法合成含有阿霉素的聚乙二醇化脂质体,其中含有天然磷脂,可以大量封装阿霉素。所得纳米粒子呈圆形,充分控制阿霉素释放(48 h后释放37%阿霉素),与游离阿霉素相比细胞渗透性增加,各种骨肉瘤细胞系(包括MG-63,U2-OS,SaOS-2)的死亡增加。因此,它们可以为骨肉瘤治疗提供新颖,更有效的治疗方法。 壳聚糖是一种天然、丰富、生物相容性好的甲壳类和昆虫寡糖化合物。与其他合成高分子化合物相比,壳聚糖是一种真正绿色和廉价的天然高分子化合物。CRISPIN的研究小组将带负电荷的寡核苷酸Dz13与带正电荷的壳聚糖和几乎电荷中性的阿霉素结合形成带正电的纳米粒子,其对负电荷的肿瘤细胞有吸引力[46-47]。与游离阿霉素相比,这些纳米粒子的治疗成功地抑制了骨肉瘤细胞的生长,这表明它们可用于骨肉瘤的进一步研究。SUN等[48]开发了一种葡聚糖-接枝-聚乙烯亚胺纳米平台,用于骨肉瘤的化疗。他们制造了葡聚糖-阿霉 素-聚乙烯亚胺纳米粒子,以实现有效的药物传递。与游离阿霉素相比,这些纳米粒子在将阿霉素递送到骨肉瘤细胞系(MG-63和Saos-2)方面具有更高的效率,同时对骨肉瘤细胞具有更高的毒性。 介孔二氧化硅纳米粒子具有生物相容性高、孔径可调、骨架稳定、表面易修饰等特点,是一种很有前景的载体材料。与孔径为2-5 nm的介孔二氧化硅纳米粒子相比,大孔介孔二氧化硅纳米粒子(介孔尺寸为6-50 nm)更有利于包封药物[49-50]。SHAHABI等[51]证明阳离子介孔二氧化硅纳米粒子不能加载阿霉素,因此对骨肉瘤细胞没有任何细胞毒性和抗增殖潜力。相反,带负电荷的,特别是磺酸盐功能化的介孔二氧化硅纳米粒子,可以加载大量的阿霉素并且在血清存在下有效进入细胞。与未功能化的介孔二氧化硅纳米粒子和游离阿霉素相比,这些装载阿霉素的功能化介孔二氧化硅纳米粒子显示出更高的细胞毒性和抗增殖作用。WANG等[52]通过自组装过程合成了皂石-聚乙二醇-聚乳酸纳米复合材料。聚乙二醇-聚乳酸的疏水性嵌段充当与载药的皂石表面结合的锚点,而亲水性聚乙二醇部分用作保护壳,其在生理条件下改善纳米复合物的稳定性。这些纳米载体可有效加载阿霉素,并显示出持续的药物释放行为。在骨肉瘤细胞系CAL-72细胞中,装载阿霉素的纳米复合物显示出比游离阿霉素更高的细胞毒性。KAMBA等[53-54]合成了一种CaCO3纳米载体用于承载抗肿瘤药物,可选择性地靶向癌细胞,有效杀死骨肉瘤细胞并且不会诱发特异性毒性。封装的阿霉素显著抑制MG-63骨肉瘤细胞,并且在pH 4.8的酸性环境中快速释放,而在生理pH 7.4下释放缓慢。BOSIO等[55]开发了一种基于λ-角叉胶和叶酸修饰的CaCO3纳米载体,用于高效负载阿霉素。λ-角叉胶-叶酸与CaCO3纳米粒子的偶联使其表面积扩大9倍并促进靶向癌细胞。 2.1.2 金属 金属纳米粒子,尤其是具有抗菌特性的纳米粒子,例如锌、铜和银,通常用于手术后的抗细菌感染。此外,纳米金属也可抑制骨肿瘤的生长[56]。NAYAK等[57]从植物树皮提取物中合成银纳米粒子。这些绿色合成的银纳米粒子不仅具有抗菌活性,而且还能通过剂量依赖性细胞毒性对抗MG-63骨肉瘤细胞,并最终通过染色质凝聚导致细胞死亡。金纳米粒子是美国食品和药物管理局批准的用于临床试验的纳米材料。大量研究显示,金纳米粒子在肿瘤诊断和治疗中具有广阔的前 景[58-60],例如一些生物分子修饰的金纳米粒子可用于骨肿瘤的靶向治疗[61]。RAHIM等[62]合成了含有糖基化产物的金纳米粒子。这些糖原性金纳米粒子对人骨肉瘤细胞系(Saos-2)具有毒性,而当其浓度小于1.0 mmol/L时,正常人胚胎肺细胞系(L-132)不受任何影响。NAIR等[63]研究了氧化锌纳米粒子对骨肉瘤细胞系(MG-63)的细胞毒性。他们认为细胞毒性可能与细胞膜上Fas上调引起的细胞凋亡有关,也可能与氧化锌粒子的尺寸、大小和表面覆盖有关。 2.1.3 钙调蛋白依赖性激酶Ⅱ型抑制剂KN-93 KN-93是一种可渗透细胞、可逆和竞争性的钙调蛋白依赖性激酶Ⅱα型(calmodulin-dependent kinase Iiα,CaMKIIα)抑制剂。富勒烯C60在肿瘤治疗中的主要用途是作为抗肿瘤试剂或纳米载体。一些研究已经证明富勒烯通过涉及氧化应激、抗血管生成、免疫调节和自噬调节多种机制表现出显著的抗肿瘤作用[65-68]。XU等[64]证实了富勒烯C60纳米晶体与CaMKIIα相互作用对骨肉瘤治疗的生物学效应,并研究了CaMKIIα抑制与自噬降解之间的关系。富勒烯C60纳米晶体在骨肉瘤细胞中可引发活性氧依赖性细胞毒性和CaMKIIα的持续活化。CaMKIIα活化导致富勒烯C60纳米晶体的细胞毒性降低。CaMKIIα抑制剂KN-93对CaMKIIα活性的抑制显著促进了富勒烯C60纳米晶体的抗骨肉瘤作用。此外,CaMKIIα活性的抑制导致溶酶体碱化和增大,并且损害溶酶体的降解功能,导致自噬体积聚。重要的是,过量的自噬体积聚和自噬降解阻断在KN-93增强骨肉瘤细胞死亡中起重要作用,见图3。 "
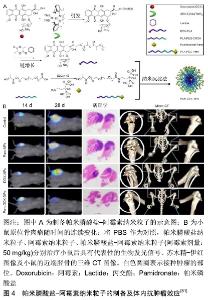
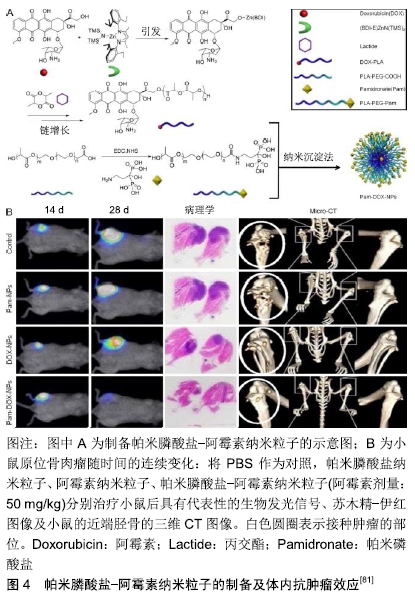
2.1.4 姜黄素 姜黄素是从姜科或天南星科中的一些植物的根茎中提取的一种化学成分。姜黄中含3%-6%的姜黄素,为二酮类化合物。研究表明,姜黄素具有抗肿瘤、抗氧化等作用。DHULE等[69]制备了姜黄素脂质体、C6神经酰胺脂质体和C6-姜黄素脂质体3种脂质体,检测了它们的抗骨肉瘤细胞株潜能。与单独使用姜黄素脂质体相比,姜黄素联合C6在MG-63和KH骨肉瘤细胞系中显示出更强的细胞毒性。重要的是,与骨肉瘤细胞系相比,C6-姜黄素脂质体对未转化的人类细胞(人间充质干细胞)毒性较小。C6-姜黄素-FA脂质体利用聚乙二醇提高血浆半衰期,并用FA标记用于体内靶向递送,可显著抑制肿瘤生长。 2.1.5 紫杉醇 紫杉醇作为一个具有抗肿瘤活性的二萜生物碱类化合物,是目前已发现的优秀的天然抗肿瘤药物,在临床上已经广泛用于乳腺癌、卵巢癌和部分头颈癌和肺癌的治疗。胶束是具有疏水核和亲水壳的自组装封闭脂质单层,广泛用作水溶性差的药物输送[41, 70-71]。ZHANG等[72]制备了可生物降解的聚磷酸酯基高分子胶束和壳交联的纳米粒子。这种高分子胶束可以有效地掺入高浓度的紫杉醇,并持续释放紫杉醇,用于治疗骨肉瘤的肺转移。 2.1.6 唑来膦酸 唑来膦酸是一种新型、高效的第三代双膦酸盐类骨吸收抑制剂,其具有高度骨亲和力,进入体内后迅速被骨组织吸收。SALZANO等[73]将唑来膦酸与磷酸钙纳米粒子结合,并用聚乙二醇化脂质体表面修饰,以开发含唑来膦酸的聚乙二醇化纳米粒子。这些纳米粒子具有均匀的粒度分布和高唑来膦酸负荷,并且它们增强了唑来膦酸对骨肉瘤细胞系的抗增殖作用。 2.1.7 阿仑膦酸盐 阿仑膦酸盐是第3代氨基双膦酸盐类骨吸收抑制剂,与骨内羟基磷灰石有强亲和力,通过抑制破骨细胞的活性而发挥抗骨吸收作用。研究人员合成了阿仑膦酸盐偶联聚乙二醇功能化的磷酸钙纳米粒子,用于骨靶向给药[74]。阿仑膦酸盐-聚乙二醇-阿仑膦酸盐修饰的磷酸钙纳米粒子粒径均匀,生物分布良好,具有良好的生物相容性和骨靶向能力,并且在酸性环境下具有更快的药物释放能力。 2.1.8 褪黑素 褪黑素是一种由哺乳动物松果体产生的胺类激素,能使皮肤色素变浅,其含量随每天的时间变化,其具有抗衰老、调节免疫、抗肿瘤等多项生理功能。聚乙交酯丙交酯是用于生物医学产品和装置的最流行的材料之一,具有优异的系统特性和生物降解性,已用于外科手术的缝合材料[75]。ALTMDAL等[76]使用乳液-扩散-蒸发法制备直径为200 nm的负载褪黑素的聚乙交酯丙交酯纳米粒子。这些纳米粒子对褪黑素的包封效率为14%,并且在40 d后释放70%的褪黑素。空白和负载于聚乙交酯丙交酯纳米粒子的褪黑素均可通过细胞摄取对MG-63骨肉瘤细胞产生毒性作用。因此,该纳米粒子包裹的褪黑素作为骨肉瘤常规化疗辅助药物具有巨大的应用前景。 2.1.9 维甲酸 维甲酸是维生素A的代谢中间产物,主要影响骨的生长和促进上皮细胞增生、分化、角质溶解等代谢作用。研究显示,将维甲酸装载在壳聚糖纳米粒中,显示出对骨肉瘤的抗肿瘤作用[77]。上述纳米粒子在酸性或生理pH环境中均具有药物的控释作用。该纳米药物可使MG-63癌细胞凋亡水平升高,并且能有效地抑制肿瘤生长。 2.1.10 米伐木肽 胞壁酰二肽是卡介苗分枝杆菌细胞壁中最小的生物活性肽聚糖成分[78]。米伐木肽是胞壁酰二肽的合成衍生物。脂质体包封米伐木肽有利于其靶向递送至单核细胞和巨噬细胞,并通过分泌白细胞介素6与肿瘤坏死因子α和增强吞噬作用激活巨噬细胞和单核细胞,从而达到杀死骨肉瘤细胞的效果[78-79]。 2.1.11 组合 吉西他滨是一种著名的抗肿瘤药物,氯法齐明是一种对肿瘤和麻风病有效的药物。CALISKAN等[80]以吉西他滨和氯法齐明共载脂质体制剂为基础,开发了一种新型的双药给药系统。与吉西他滨或氯法齐明共载脂质体相比,吉西他滨/氯法齐明共载脂质体对骨肉瘤细胞的细胞毒性明显增强,证明了氯法齐明对骨肉瘤细胞的毒性及与吉西他滨的协同作用。因此,吉西他 滨/氯法齐明共载脂质体传递系统被认为是一种治疗骨肉瘤的新方法。 为了达到靶向治疗骨肉瘤的目的,ZHAO等[82]开发了新型紫杉醇纳米粒子,其涂有聚多巴胺,并由阿仑膦酸盐作为配体靶向骨肉瘤。聚多巴胺在碱性环境中形成薄的聚多巴胺膜,这为没有官能团的化合物提供了平台以进行二次反应。研究发现靶向纳米粒子在血浆中稳定并显示出持续的药物释放行为,体内研究表明其在肿瘤中的积累比非靶向纳米粒子更多,并且与其他治疗组相比获得了更好的治疗效果,显著降低了紫杉醇的不良反应。因此,紫杉醇-聚多巴胺-阿仑膦酸盐纳米粒子可为骨肉瘤靶向治疗提供可行且有效的策略。YIN等[81]研制了聚乳酸纳米粒子负载阿霉素,并包覆骨靶向帕米膦酸盐用于恶性骨肿瘤的靶向治疗。他们通过阿霉素-聚乳酸高分子、聚乙二醇-聚乳酸和聚乳酸-聚乙二醇-帕米膦酸盐共沉淀法来制备帕米膦酸盐-阿霉素纳米粒子。与盐水治疗组相比,帕米膦酸盐纳米粒子组和阿霉素纳米粒子组略微减缓原发肿瘤生长,但在接受帕米膦酸盐-阿霉素纳米粒子的小鼠中,可见肿瘤体积明显消退。在接受盐水、帕米膦酸盐纳米粒子和阿霉素纳米粒子治疗的荷瘤小鼠右胫骨近端,明显存在与骨肉瘤进展相关的严重病理性骨破损,而帕米膦酸盐-阿霉素纳米粒子组显著减弱了骨肉瘤的病理性骨破损进展,见图4。 "
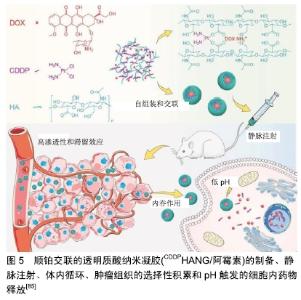
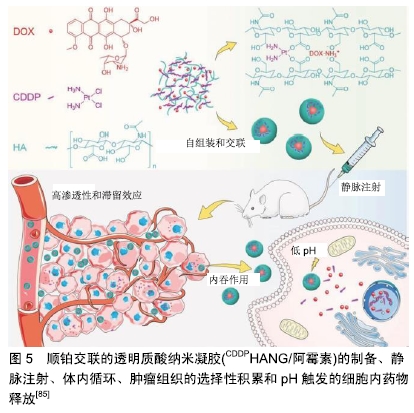
树枝状大分子是一种具有树形结构的高分子,其含有超支化亚基和源自中心核的末端基团。CLEMENTI等[83]合成了一种聚乙二醇-树枝状大分子作为紫杉醇和阿仑膦酸盐联合递送的载体。紫杉醇-聚乙二醇-阿仑膦酸盐树枝状大分子通过与骨矿物羟基磷灰石结合表现出对骨靶向的极大亲和力,并通过延长半衰期改善了药代动力学特征。SEGAL等[84]用N-(2-羟丙基)甲基丙烯酰胺共聚物结合阿仑膦酸盐和强效抗血管生成剂TNP-470。在骨肉瘤模型中,与游离阿仑膦酸盐和TNP-470联合使用相比,聚N-(2-羟丙基)甲基丙烯酰 胺-阿仑膦酸盐-TNP-470显示出具有更好的抗血管生成和抗肿瘤活性。 高分子纳米凝胶是指由亲水性高分子或两亲高分子形成的纳米级交联型三维网络结构,其在药物载体领域有着重要的应用。Ding团队制备顺铂(cisplatin, CDDP)交联的透明质酸纳米凝胶(CDDPHANG),用于有效递送阿霉素以治疗骨肉瘤[85]。由于纳米凝胶的稳定性增强,与游离药物相比,CDDP交联用于负载阿霉素的纳米药物(CDDPHANG/阿霉素)循环时间明显延长。此外,在肿瘤部位有效积累后,阿霉素和CDDP同步释放到肿瘤细胞内酸性环境中。基于阿霉素和CDDP的协同诱导凋亡作用,与其他组合相比,CDDPHANG/阿霉素显示出明显增强的抗肿瘤效力,表明其对骨肉瘤化疗具有巨大的前景,见图5。 "
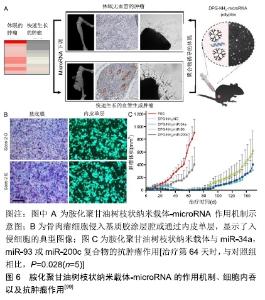
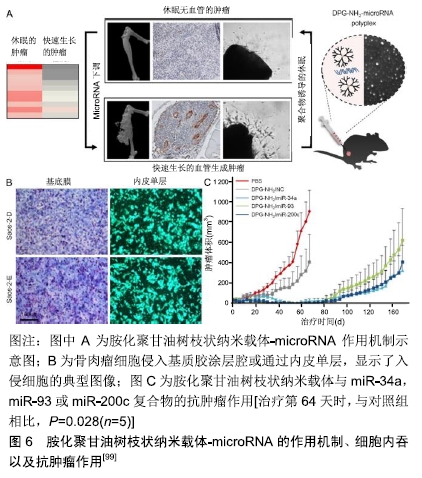
甲氨蝶呤为抗叶酸类抗肿瘤药,通过抑制二氢叶酸还原酶达到阻碍肿瘤细胞DNA的合成,从而抑制肿瘤细胞的生长与繁殖。PRASAD等[86]以羟基磷灰石纳米颗粒为核心,以聚乙烯醇为冠状结构,开发了用于甲氨蝶呤和吉西他滨双重递送的纳米羟基磷灰石纳米颗粒。MG-63骨肉瘤细胞的细胞活力研究证实,高分子接枝的纳米粒子是无细胞毒性的,而载药的纳米粒子表现出剂量依赖性的细胞毒性。LI等[87]首次在原位骨肿瘤模型中应用了一种基于Ag2S量子点的多功能纳米药物,用于化疗和抑制骨溶解。将阿仑膦酸盐偶联到Ag2S量子点表面进行骨靶向药物递送和骨溶解抑制,同时将阿霉素包裹在围绕Ag2S量子点的疏水层中进行肿瘤化疗。这种阿仑膦酸盐/阿霉素@Ag2S制剂抑制破骨细胞形成和促进成骨分化,显示出预期降低癌相关骨溶解的作用。 2.2 蛋白类药 曲妥珠单抗是抗人类表皮生长因子受体2(human epidermal growth factor receptor-2, HER2)的单克隆抗体,通过附着在HER2上来阻止人体表皮生长因子在HER2上的附着,从而阻断癌细胞的生长,还可以刺激身体的免疫细胞去清除癌细胞。HER2在骨肉瘤中过度表达使HER2成为潜在的治疗靶点[88-90]。然而,研究表明骨肉瘤细胞对抗HER2抗体曲妥珠单抗疗法无反应[91]。LI等[92]建立了稳定的、非共价的曲妥珠单抗与纳米材料氧化石墨烯结合,生成多价曲妥珠单抗/氧化石墨烯复合物。该复合物与HER2的结合显著增强,并快速杀伤骨肉瘤细胞。静脉滴注曲妥珠单抗/氧化石墨烯可以根除免疫缺陷小鼠体内已建立的异种移植骨肉瘤,使动物存活时间延长,而原形态的曲妥珠单抗无此疗效。KOVACH等[93]提出一种体外小鼠模型,用于初步研究氧化铁纳米粒子与血管内皮生长因子抗体和CD80配体的有效结合,最终应用于人骨肉瘤治疗。直径35 nm的氧化铁磁性纳米粒子与N-羟基丁二酰亚胺生物相容涂层官能团化,并在表面与血管内皮生长因子抗体和CD80配体结合。这些蛋白结合后具有靶向骨肉瘤细胞并诱导细胞凋亡的能力。结果表明,CD80配体和血管内皮生长因子抗体联合应用在24 h和72 h内均能有效降低骨肉瘤细胞异常增殖。 2.3 核酸类药 治疗或预防恶性肿瘤的另一种方法是抑制肿瘤起始细胞向肿瘤细胞的分化或者敲除导致恶性表型发展的特定基因。然而,因为RNA/DNA分子在递送过程中易于降解以及细胞穿透受阻的问题,导致这种方法很难达到治疗效果[94]。纳米技术通过提供运输RNA/DNA分子的载体或影响肿瘤细胞基因表达过程,可能为实现这一目标提供了重要机会[95]。 2.3.1 小干扰RNA(small interfering RNA, siRNA) siRNA是一类双链RNA分子,长度为20-25个碱基对,它可以干扰特定基因转录后降解的mRNA,从而防止翻译。XIONG等[96]制备了直径为150 nm、介孔尺寸为12 nm的胺功能化核壳二氧化硅纳米粒子。该纳米粒子表面积大,siRNA载量高,在外界磁场作用下表现出磁性反应能力。这些纳米粒子通过单宁酸修饰以改善其分散稳定性,并作为pH响应释放开关。体外结果显示胺功能化核壳磁性二氧化硅纳米粒子可以将功能性siRNA导入人骨肉瘤细胞中,表明这些纳米粒子可能为siRNA治疗骨肉瘤提供很好的平台。YU等[97]开发了直径为10 nm的聚乙烯亚胺功能化混合二氧化硅粒子,该粒子能够极好的向骨肉瘤细胞递送siRNA,与传统试剂相比具有显著的抑制作用。研究人员还制造了一种病毒感染的高分子模拟纳米载体,用来传递siRNA,特异性敲除骨肉瘤细胞系(U-2OS)里的基因。这种方法可以特异性地杀死癌细胞,而无需额外使用毒性化疗药物[98]。尽管siRNA在上述骨肉瘤研究中得到青睐,但短半衰期和快速排泄率限制了它们的发展应用。这需要更加智能的药物输送系统将其递送至骨肉瘤部位。 2.3.2 微小RNA(microRNA, miRNA) miRNA是一种长度为21-23个碱基的单链小分子RNA,由具有发夹结构的单链RNA前体经过Dicer酶加工后生成,调节基因的表达。TIRAM等[99]开发了一种胺化聚甘油树枝状纳米载体,用于靶向骨癌并递送miRNA。与正常骨相比,骨肉瘤患者肿瘤样本中的miRNA下调。用胺化聚甘油树枝状纳米载体-miRNA治疗后骨肉瘤的血管生成能力减弱,肿瘤休眠时间延长。研究人员评估了Saos-2-D和Saos-2-E细胞的体外致瘤潜能,发现它们的增殖率差异无显著性意义,同时2种细胞系通过基质凝胶或内皮单层侵袭和向血清迁移的能力也相似。胺化聚甘油树枝状纳米载体-miR-34a,胺化聚甘油树枝状纳米载体-miR-93和胺化聚甘油树枝状纳米载体-miR-200c复合物都能够将Saos-2-E肿瘤转化为类似休眠肿瘤的表型。当停止治疗后,这种休眠状态持续40 d,然后发生肿瘤逃逸,见图6。虽然前景广阔,但树枝状大分子仍然存在一些问题,比如它们的制备比其他纳米粒子更昂贵,合成步骤更多,这对于大规模生产和临床应用是个挑战。 "
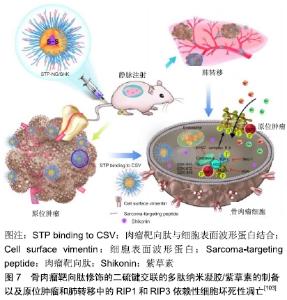
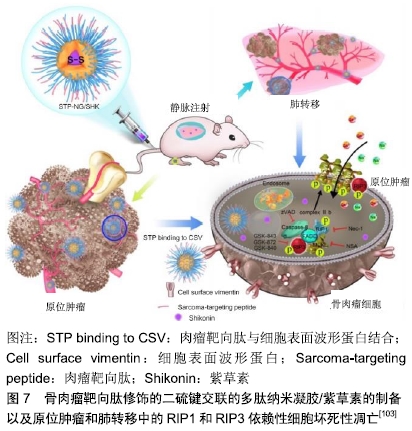
2.3.3 短发夹RNA(short hairpin RNA,shRNA) shRNA是一段具有紧密发卡环的RNA序列,常被用于沉默靶基因的表达。研究人员利用单甲氧基聚乙二醇-壳聚糖纳米粒子作为载体,以shRNA转染MG-63骨肉瘤细胞对抗livin和survivin基因,并评价其对细胞增殖和凋亡的影响[100]。结果显示单甲氧基聚乙二醇-壳聚糖搭载的shRNA通过同时敲除livin和survivin基因,可以抑制MG-63细胞的增殖并促进其凋亡。 2.4 联合治疗 对于骨肉瘤肺转移,载有阿霉素的纳米脂质载体和针对Bcl-2、P-糖蛋白的siRNA结合是一种潜在的治疗方式,并通过向该复合物中添加促黄体生成素释放激素的合成类似物,实现向肺部的递送[101]。HAGHIRALSADAT等[102]合成了双靶向(细胞外抗EphA2和细胞内抗JNK1)、阿霉素负载脂质体用于骨肉瘤转移性疾病的治疗,其中YSA肽与二硬脂酰基磷脂酰乙醇胺-聚乙二醇结合实现EphA2的靶向作用,然后将阿霉素和JIP1 siRNA装载于囊泡内。该纳米粒子为单分散球形结构,平均直径约为100 nm,具有良好的结构特征,表面光滑。在使用靶向系统(YSA-L-siRNA-阿霉素)时,骨肉瘤细胞的摄取比例比非靶向系统(L-siRNA-阿霉素)更多。Ding团队开发了骨肉瘤靶向肽修饰的二硫键交联的多肽纳米凝胶,用于细胞内递送紫草素(一种草药提取物),并以最小的全身毒性抑制骨肉瘤进展[103]。实验结果显示骨肉瘤靶向肽修饰的二硫键交联的多肽纳米凝胶/紫草素表现出优异的抗肿瘤和抑制肺转移效力。同时,骨肉瘤靶向肽修饰的二硫键交联的多肽纳米凝胶/紫草素显示出全身的低毒性,见图7。 "
| [1] MIRABELLO L, TROISI RJ, SAVAGE SA. Osteosarcoma Incidence and Survival Rates From 1973 to 2004 Data From the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531-1543. [2] IANNACI G, LUISE R, SAPERE P, et al. Extraskeletal osteosarcoma: A very rare case report of primary tumor of the colon-rectum and review of the literature. Pathol Res Pract. 2013; 209(6):393-396. [3] MARTIN JW, SQUIRE JA, ZIELENSKA M. The genetics of osteosarcoma. Sarcoma. 2012;2012:627254-627254. [4] WANG LL. Biology of osteogenic sarcoma. Cancer J. 2005; 11(4):294-305. [5] KANSARA M, THOMAS DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26(1):1-18. [6] SMELAND S, MULLER C, ALVEGARD TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488-494. [7] PAKOS EE, NEARCHOU AD, GRIMER RJ, et al. Prognostic factors and outcomes for osteosarcoma: An international collaboration. Eur J Cancer. 2009;45(13):2367-2375. [8] HARTING MT, LALLY KP, ANDRASSY RJ, et al. Age as a prognostic factor for patients with osteosarcoma: an analysis of 438 patients. J Cancer Res Clin. 2010;136(4):561-570. [9] ALESSANDRA L, ANDREA P, ALESSANDRO C, et al. Height as a risk factor for osteosarcoma. J Pediatr Hematol Oncol. 2005; 27(6):314-318. [10] SUBBIAH V, MADSEN VS, RAYMOND AK, et al. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporosis Int. 2010;21(6):1041-1045. [11] BASSIN EB, WYPIJ D, DAVIS RB, et al. Age-specific fluoride exposure in drinking water and osteosarcoma (United States). Cancer Cause Control. 2006;17(4):421-428. [12] GANDHI D, NAOGHARE PK, BAFANA A, et al. Fluoride-Induced Oxidative and Inflammatory Stress in Osteosarcoma Cells: Does It Affect Bone Development Pathway? Biol Trace Elem Res. 2017; 175(1):103-111. [13] KIM FM, HAYES C, WILLIAMS PL, et al. An Assessment of Bone Fluoride and Osteosarcoma. J Dent Res. 2011;90(10):1171-1176. [14] LEVY M, LECLERC BS. Fluoride in drinking water and osteosarcoma incidence rates in the continental United States among children and adolescents. Cancer Epidemiol. 2012;36(2): E83-E88. [15] ARCHER NP, NAPIER TS, VILLANACCI JF. Fluoride exposure in public drinking water and childhood and adolescent osteosarcoma in Texas. Cancer Cause Control. 2016;27(7): 863-868. [16] CHOU AJ, GELLER DS, GORLICK R. Therapy for osteosarcoma: where do we go from here? Paediatr Drugs. 2008;10(5):315-327. [17] SCHWARZ R, BRULAND O, CASSONI A, et al. The Role of Radiotherapy in Oseosarcoma. Cancer Treat Res. 2009;152: 147-164. [18] ERRANI C, LONGHI A, ROSSI G, et al. Palliative therapy for osteosarcoma. Expert Rev Anticanc. 2011;11(2):217-227. [19] YASKO AW. Surgical Management of Primary Osteosarcoma. Cancer Treat Res. 2009;152:125-145. [20] BIELACK S, JURGENS H, JUNDT G, et al. Osteosarcoma: The COSS Experience. Cancer Treat Res. 2009;152:289-308. [21] JAFFE N. Osteosarcoma: Review of the Past, Impact on the Future. The American Experience. Cancer Treat Res. 2009; 152:239-262. [22] MARULANDA GA, HENDERSON ER, JOHNSON DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20. [23] TA H, DASS C, CHOONG P, et al. Osteosarcoma treatment: state of the art. Cancer Metast Rev. 2009;28(1-2):247-263. [24] HATTINGER CM, PASELLO M, FERRARI S, et al. Emerging drugs for high-grade osteosarcoma. Expert Opin Emerg Dr. 2010;15(4):615-634. [25] GELLER DS, GORLICK RJCAHO. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8(10):705-718. [26] CARRLE D, BIELACK SS. Current strategies of chemotherapy in osteosarcoma. Int Orthop. 2006;30(6):445-451. [27] SMELAND S, BRULAND OS, HJORTH L, et al. Results of the Scandinavian Sarcoma Group XIV protocol for classical osteosarcoma 63 patients with a minimum follow-up of 4 years. Acta Orthopaedica. 2011;82(2):211-216. [28] DELANEY TF, PARK L, GOLDBERG SI, et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol. 2005;61(2): 492-498. [29] SIKORA K. The impact of future technology on cancer care. Clin Med. 2002;2(6):560-568. [30] CHIDAMBARAM M, MANAVALAN R, KATHIRESAN KJJOP, et al. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci. 2011;14(1):67-77. [31] SAKAMOTO JH, VAN DE VEN AL, GODIN B, et al. Enabling individualized therapy through nanotechnology. Pharmacol Res. 2010;62(2):57-89. [32] XUEYUAN G, JIANXUN D, ZHIYU Z, et al. Polymeric Nanocarriers for Drug Delivery in Osteosarcoma Treatment. Curr Pharm Des. 2015;21(36):5187-5197. [33] MA H, JIANG W, DING J, et al. Polymer Nanoparticle-Based Chemotherapy for Spinal Malignancies. J Nanomater. 2016; 2016(2):1-14. [34] BINKHATHLANAB Z, BROCKS DR, LAVASANIFARAC AJEJOP, et al. Encapsulation of P-glycoprotein inhibitors by polymeric micelles can reduce their pharmacokinetic interactions with doxorubicin. Eur J Pharm Biopharm. 2012;81(1):142-148. [35] ANBARASAN B, BABU SV, ELANGO K, et al. pH Responsive Release of Doxorubicin to the Cancer Cells by Functionalized Multi-Walled Carbon Nanotubes. J Nanosci Nanotechnol. 2015; 15(7):4799-4805. [36] TANG X, LIANG Y, FENG X, et al. Co-delivery of docetaxel and Poloxamer 235 by PLGA–TPGS nanoparticles for breast cancer treatment. Mater Sci Eng C Mater Biol Appl. 2015;49:348-355. [37] WANG H, LIU Z, GOU Y, et al. Apoptosis and necrosis induced by novel realgar quantum dots in human endometrial cancer cells via endoplasmic reticulum stress signaling pathway. Int J Nanomedicine. 2015;10:5505-5512. [38] YIN-JIA C, GUO-FENG L, JING-YI Z, et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl Mater Interfaces. 2015;7(17):9078-9087. [39] ZHAO X, QI C, LI Y, et al. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharm Biopharm. 2015;93:27-36. [40] NAIARA MV, ENRIC X, PATRICIA J, et al. The oncolytic adenovirus Δ24-RGD in combination with cisplatin exerts a potent anti-osteosarcoma activity. J Bone Miner Res. 2015;29(10): 2287-2296. [41] FANG Z, SUN Y, XIAO H, et al. Targeted osteosarcoma chemotherapy using RGD peptide-installed doxorubicin-loaded biodegradable polymeric micelle. Biomed Pharmacother. 2017; 85:160-168. [42] DING J, LI C, ZHANG Y, et al. Chirality-mediated polypeptide micelles for regulated drug delivery. Acta Biomater. 2015; 11(1):346-355. [43] KROON J, BUIJS JT, VAN DHG, et al. Liposomal delivery of dexamethasone attenuates prostate cancer bone metastatic tumor growth In Vivo. Prostate. 2015;75(8):815-824. [44] MITXELENA-IRIBARREN O, HISEY CL, ERRAZQUIN-IRIGOYEN M, et al. Effectiveness of nanoencapsulated methotrexate against osteosarcoma cells: in vitro cytotoxicity under dynamic conditions. Biomed Microdevices. 2017;19(2):35. [45] HAGHIRALSADAT F, AMOABEDINY G, SHEIKHHA MH, et al. A Novel Approach on Drug Delivery: Investigation of A New Nano-Formulation of Liposomal Doxorubicin and Biological Evaluation of Entrapped Doxorubicin on Various Osteosarcoma Cell Lines. Cell J. 2017;19(Suppl 1):55-65. [46] MEI LIN T, FRIEDHUBER AM, DASS CR, et al. Co-nanoencapsulated doxorubicin and Dz13 control osteosarcoma progression in a murine model. J Pharm Pharmacol. 2012;65(1):35-43. [47] FRIEDHUBER AM, VIJAY C, SOMKAMON M, et al. Nucleotropic doxorubicin nanoparticles decrease cancer cell viability, destroy mitochondria, induce autophagy and enhance tumour necrosis. J Pharm Pharmacol. 2015;67(1):68-77. [48] SUN K, WANG J, ZHANG J, et al. Dextran-g-PEI nanoparticles as a carrier for co-delivery of adriamycin and plasmid into osteosarcoma cells. Int J Biol Macromol. 2011;49(2):173-180. [49] JI-HO P, LUO G, GEOFFREY VM, et al. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009;8(4):331-336. [50] SHAO D, LU MM, ZHAO YW, et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017;49:531-540. [51] SHAKIBA S, SVEA DS, TOBIAS B, et al. Enhancing Cellular Uptake and Doxorubicin Delivery of Mesoporous Silica Nanoparticles via Surface Functionalization: Effects of Serum. ACS Appl Mater Interfaces. 2015;7(48):26880-26891. [52] WANG G, MACIEL D, WU Y, et al. Amphiphilic polymer-mediated formation of laponite-based nanohybrids with robust stability and pH sensitivity for anticancer drug delivery. ACS Appl Mater Interfaces. 2014;6(19):16687-16695. [53] KAMBA SA, ISMAIL M, HUSSEIN-AL-ALI SH, et al. In Vitro Delivery and Controlled Release of Doxorubicin for Targeting Osteosarcoma Bone Cancer. Molecules. 2013;18(9):10580-10598. [54] KAMBA AS, ISMAIL M, IBRAHIM TAT, et al. In Vitro Ultrastructural Changes of MCF-7 for Metastasise Bone Cancer and Induction of Apoptosis via Mitochondrial Cytochrome C Released by CaCO3/Dox Nanocrystals. Biomed Res Int. 2014;2014:391869. [55] BOSIO VE, CACICEDO ML, CALVIGNAC B, et al. Synthesis and characterization of CaCO3-biopolymer hybrid nanoporous microparticles for controlled release of doxorubicin. Colloids Surf B Biointerfaces. 2014;123:158-169. [56] DÍEZ P, GONZÁLEZ-MUÑOZ M, GONZÁLEZ-GONZÁLEZ M, et al. Functional insights into the cellular response triggered by a bile-acid platinum compound conjugated to biocompatible ferric nanoparticles using quantitative proteomic approaches. Nanoscale. 2017;9(28):9960-9972. [57] NAYAK D, ASHE S, RAUTA PR, et al. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater Sci Eng C Mater Biol Appl. 2016;58(30):44-52. [58] DEENDAYAL M, AVUDAIPPAN M, YASZEMSKI MJ, et al. Cellular uptake of gold nanoparticles directly cross-linked with carrier peptides by osteosarcoma cells. J Mater Sci Mater Med. 2009; 20(1):347-350. [59] WU J, LIU B, WU H, et al. A Gold Nanoparticle Platform for the Delivery of Functional TGF-β1 siRNA Into Cancer Cells. J Biomed Nanotechnol. 2016;12(4):800-810. [60] WU C, CHEN H, WU X, et al. The influence of tumor-induced immune dysfunction on the immune cell distribution of gold nanoparticles in vivo. Biomater Sci. 2017;5(8):1531-1536. [61] BRAZZALE C, MASTROTTO F, MOODY P, et al. Control of targeting ligand display by pH-responsive polymers on gold nanoparticles mediates selective entry into cancer cells. Nanoscale. 2017;9(31):11137-11147. [62] RAHIM M, IRAM S, KHAN MS, et al. Glycation-assisted synthesized gold nanoparticles inhibit growth of bone cancer cells. Colloids Surf B Biointerfaces. 2014;117(9):473-479. [63] NAIR S, SASIDHARAN A, DIVYA RANI VV, et al. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J Mater Sci Mater Med. 2009; 20(1):235-241. [64] XU J, WANG H, HU Y, et al. Inhibition of CaMKIIα Activity Enhances Antitumor Effect of Fullerene C60 Nanocrystals by Suppression of Autophagic Degradation. Adv Sci (Weinh). 2019: 1801233. [65] TZIRAKIS MD, ORFANOPOULOS MJCR. Radical reactions of fullerenes: from synthetic organic chemistry to materials science and biology. Chem Rev. 2013;113(7):5262-5321. [66] MENG H, XING G, SUN B, et al. Potent angiogenesis inhibition by the particulate form of fullerene derivatives. ACS Nano. 2010;4(5): 2773-2783. [67] YANG D, ZHAO Y, GUO H, et al. [Gd@ C82 (OH) 22] n nanoparticles induce dendritic cell maturation and activate Th1 immune responses. ACS Nano. 2010;4(2):1178-1186. [68] ZHANG Q, YANG W, MAN N, et al. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy. 2009;5(8):1107-1117. [69] DHULE SS, PATRICE P, JIBAO H, et al. The combined effect of encapsulating curcumin and C6 ceramide in liposomal nanoparticles against osteosarcoma. Mol Pharm. 2014;11(2):417-427. [70] LIU L, MA G, ZHANG C, et al. An Activatable Theranostic Nanomedicine Platform Based on Self-Quenchable Indocyanine Green-Encapsulated Polymeric Micelles. J Biomed Nanotechnol. 2016;12(6):1223-1233. [71] ZHOU H, WANG G, LU Y, et al. Bio-inspired cisplatin nanocarriers for osteosarcoma treatment. Biomater Sci. 2016;4(8):1212-1218. [72] ZHANG F, ZHANG S, POLLACK SF, et al. Improving paclitaxel delivery: in vitro and in vivo characterization of PEGylated polyphosphoester-based nanocarriers. J Am Chem Soc. 2015; 137(5):2056-2066. [73] SALZANO G, MARRA M, PORRU M, et al. Self-assembly nanoparticles for the delivery of bisphosphonates into tumors. Int J Pharm. 2011;403(1-2):292-297. [74] CHU W, HUANG Y, YANG C, et al. Calcium phosphate nanoparticles functionalized with alendronate-conjugated polyethylene glycol (PEG) for the treatment of bone metastasis. Int J Pharm. 2016;516(1-2):352-363. [75] DEEPAK C, NEERAJ KJPR. BSA-PLGA-based core-shell nanoparticles as carrier system for water-soluble drugs. Pharm Res. 2013;30(9):2396-2409. [76] ALTINDAL DÇ, GÜMÜŞDERELIOĞLU M. Melatonin releasing PLGA micro/nanoparticles and their effect on osteosarcoma cells. Journal of Microencapsulation. J Microencapsul. 2016;33(1): 53-63. [77] QIN YG, ZHU LY, WANG CY, et al. Glycol chitosan incorporated retinoic acid chlorochalcone (RACC) nanoparticles in the treatment of Osteosarcoma. Lipids Health Dis. 2015;14(1):1-10. [78] ANDO K, HEYMANN MF, STRESING V, et al. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers (Basel). 2013;5(2):591-616. [79] FRAMPTON JE. Mifamurtide: a review of its use in the treatment of osteosarcoma. Paediatr Drugs. 2010;12(3):141-153. [80] CALISKAN Y, DALGIC AD, GEREKCI S, et al. A new therapeutic combination for osteosarcoma: Gemcitabine and Clofazimine co-loaded liposomal formulation. Int J Pharm. 2019;557:97-104. [81] YIN Q, TANG L, CAI KM, et al. Pamidronate functionalized nanoconjugates for targeted therapy of focal skeletal malignant osteolysis. Proc Natl Acad Sci U S A. 2016;113(32):E4601-E4609. [82] ZHAO L, BI D, QI X, et al. Polydopamine-based surface modification of paclitaxel nanoparticles for osteosarcoma targeted therapy. Nanotechnology. 2019;30(25):255101. [83] CLEMENTI C, MILLER K, MERO A, et al. Dendritic poly(ethylene glycol) bearing paclitaxel and alendronate for targeting bone neoplasms. 2011;8(4):1063-1072. [84] SEGAL E, PAN H, BENAYOUN L, et al. Enhanced anti-tumor activity and safety profile of targeted nano-scaled HPMA copolymer-alendronate-TNP-470 conjugate in the treatment of bone malignances. Biomaterials. 2011;32(19):4450-4463. [85] ZHANG Y, WANG F, LI M, et al. Self-Stabilized Hyaluronate Nanogel for Intracellular Codelivery of Doxorubicin and Cisplatin to Osteosarcoma. Adv Sci. 2018;5(5):1700821. [86] PRASAD SR, JAYAKRISHNAN A, KUMAR TS. Hydroxyapatite- poly(vinyl alcohol) core-shell nanoparticles for dual delivery of methotrexate and gemcitabine for bone cancer treatment. J Drug Deliv Sci Technol. 2019;51:629-638. [87] LI C, ZHANG Y, CHEN G, et al. Engineered Multifunctional Nanomedicine for Simultaneous Stereotactic Chemotherapy and Inhibited Osteolysis in an Orthotopic Model of Bone Metastasis. Adv Mater. 2017;29(13):1605754. [88] FLINT AF, U'REN L, LEGARE ME, et al. Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet Pathol. 2004;41(3):291-296. [89] HUGHES DP, THOMAS DG, GIORDANO TJ, et al. Cell surface expression of epidermal growth factor receptor and Her-2 with nuclear expression of Her-4 in primary osteosarcoma. Cancer Res. 2004;64(6):2047-2053. [90] GILBERTSON RJ. ERBB2 in pediatric cancer: innocent until proven guilty. Oncologist. 2005;10(7):508-517. [91] DAVID E, PAUL M, HOLCOMBE G, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children's oncology group. J Clin Oncol. 2012;30(20):2545-2551. [92] LI L, LUO CK, SONG ZW, et al. Association of anti-HER2 antibody with graphene oxide for curative treatment of osteosarcoma. Nanomedicine. 2018;14(2):581-593. [93] KOVACH AK, GAMBINO JM, NGUYEN V, et al. Prospective Preliminary In Vitro Investigation of a Magnetic Iron Oxide Nanoparticle Conjugated with Ligand CD80 and VEGF Antibody As a Targeted Drug Delivery System for the Induction of Cell Death in Rodent Osteosarcoma Cells. Biores Open Access. 2016;5(1):299-307. [94] BRUMMELKAMP TR, BERNARDS R, AGAMI R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296(5567):550-553. [95] SUSA M, MILANE L, AMIJI MM, et al. Nanoparticles: a promising modality in the treatment of sarcomas. Pharm Res. 2011;28(2): 260-272. [96] XIONG L, BI J, TANG Y, et al. Magnetic Core-Shell Silica Nanoparticles with Large Radial Mesopores for siRNA Delivery. Small. 2016;12(34):4735-4742. [97] YU M, NIU Y, YANG Y, et al. An approach to prepare polyethylenimine functionalized silica-based spheres with small size for siRNA delivery. ACS Appl Mater Interfaces. 2014;6(18): 15626-15631. [98] GU W, JIA Z, TRUONG NP, et al. Polymer nanocarrier system for endosome escape and timed release of siRNA with complete gene silencing and cell death in cancer cells. Biomacromolecules. 2013;14(10):3386-3389. [99] TIRAM G, SEGAL E, KRIVITSKY A, et al. Identification of Dormancy-Associated MicroRNAs for the Design of Osteosarcoma-Targeted Dendritic Polyglycerol Nanopolyplexes. ACS Nano. 2016;10(2):2028-2045. [100] GUAN HP, SUN JZ, FENG XL, et al. Effects of RNA interference-mediated knockdown of livin and survivin using monomethoxypolyethylene glycol-chitosan nanoparticles in MG-63 osteosarcoma cells. Mol Med Rep. 2016;13(2):1821-1826. [101] TARATULA O, KUZMOV A, SHAH M, et al. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171(3):349-357. [102] HAGHIRALSADAT F, AMOABEDINY G, NADERINEZHAD S, et al. Codelivery of doxorubicin and JIP1 siRNA with novel EphA2-targeted PEGylated cationic nanoliposomes to overcome osteosarcoma multidrug resistance. Int J Nanomedicine. 2018; 13(5):3853-3866. [103] LI SY, TAO Z, XU WG, et al. Sarcoma-Targeting Peptide-Decorated Polypeptide Nanogel Intracellularly Delivers Shikonin for Upregulated Osteosarcoma Necroptosis and Diminished Pulmonary Metastasis. Theranostics. 2018;8(5):1361-1375. [104] ASNAFI AA, BEHZAD MM, GHANAVAT M, et al. Singe nucleotide polymorphisms in osteosarcoma: Pathogenic effect and prognostic significance. Exp Mol Pathol. 2018;106:63-77. [105] PO KUEI W, MING CW, FONG CC, et al. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39(8):514-522. [106] LUSSIER DM, O'NEILL L, NIEVES LM, et al. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J Immunother. 2015;38(3):96-106. [107] GUIX M, MAYORGA-MARTINEZ CC, MERKOÇI A. Nano/micromotors in (bio) chemical science applications. Chem Rev. 2014;114(12):6285-6322. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Li Li, Ma Li. Immobilization of lactase on magnetic chitosan microspheres and its effect on enzymatic properties [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 576-581. |
| [6] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [7] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [8] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [9] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [10] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [11] | He Jie, Chang Qi. Biological reconstruction of large bone defects after resection of malignant tumor of extremities [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 420-425. |
| [12] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [13] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [14] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [15] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||