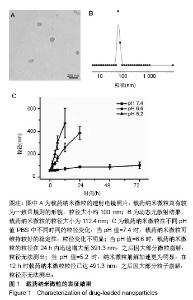
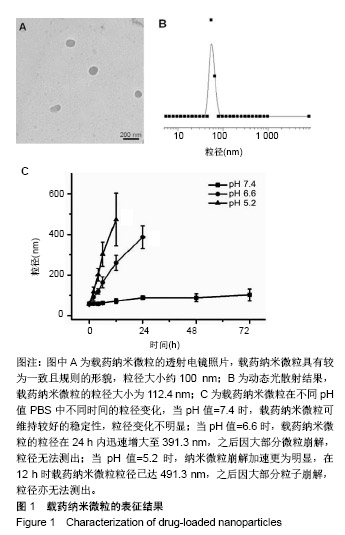
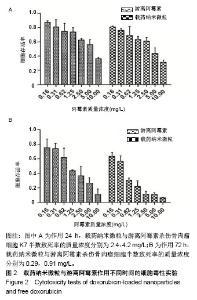
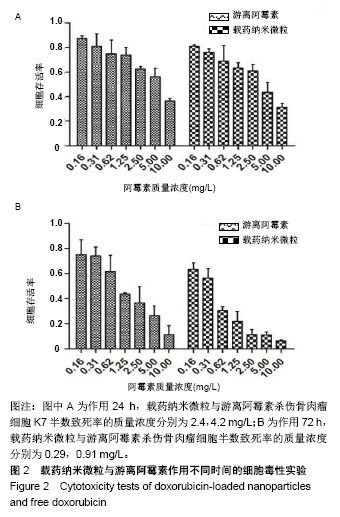
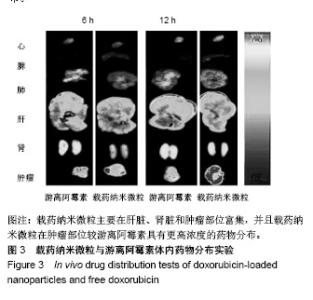
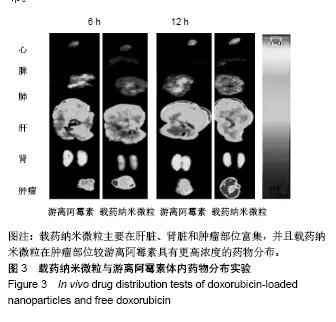
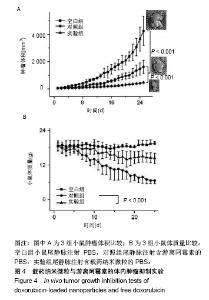
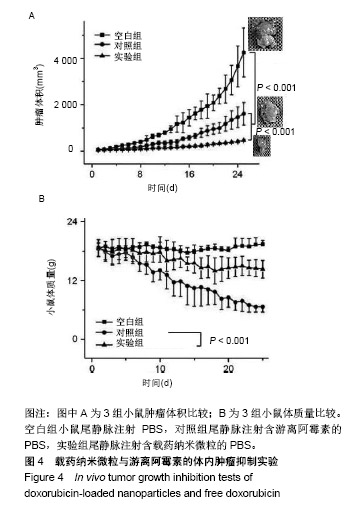
| [1]Isakoff MS, Bielack SS, Meltzer P, et al.Osteosarcoma: Current Treatment and a Collaborative Pathway to Success.J Clin Oncol. 2015;33(27):3029-3035.[2]Miao J,Wu S,Peng Z,et al.MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumour Biol. 2013;34(4):2093-2098.[3]Farfalli GL,Albergo JI,Lobos PA,et al.[Osteosarcoma lung metastases. Survival after chemotherapy and surgery]. Medicina (B Aires).2015;75(2):87-90.[4]Parry MC,Laitinen M,Albergo J,et al.Osteosarcoma of the pelvis. Bone Joint J.2016; 98-B(4):555-563.[5]Friebele JC,Peck J,Pan X,et al.Osteosarcoma: A Meta-Analysisand Review of the Literature.Am J Orthop. 2015;44(12):547-553.[6]杨丽军,禇丽萍,王婧,等.阿霉素修饰纳米银的制备及其体外抗肿瘤活性研究[J].天津医药,2018,46(5): 499-504.[7]刘永刚,杨荣松,吴红方,等.紫杉醇联合顺铂通过Wnt/β-catenin信号通路抑制鼻咽癌肿瘤干细胞增殖并促进其凋亡[J].中国组织工程研究, 2016,20(41):6118-6124.[8]王春燕,汤丽娜,祁伟祥,等.伽玛刀治疗40例骨肉瘤肺转移患者的生存分析[J].中华肿瘤防治杂志,2015, 22(8):633-636.[9]林鹏,王炜,黄伟波,等.不同化疗方案治疗成人初治非M3型AML的完全缓解率及不良反应[J].中国实验血液学杂志, 2018,26(2):422-426.[10]陈国强,左瑜芳,王镇南,等.奈达铂、顺铂在局部晚期宫颈癌同步放化疗疗效及不良反应分析[J].中国现代药物应用, 2018,1(5):82-84.[11]马岚,李冰,陈建平,等.纳米技术在药物领域中的应用[J].内蒙古医科大学学报,2018,40(5):536-540.[12]刘艳红,周建平,霍美蓉.肿瘤微环境响应型智能纳米药物载体的研究进展[J].中国药科大学学报,2016, 47(2):125-133.[13]于东,王晓欣,姜如娇.纳米抗肿瘤药物载体的研究进展[J].肿瘤, 2018, 38(6):603-609.[14]施肇源,柳甜,彭津津,等.香辛料保鲜液与壳聚糖魔芋葡甘聚糖复合膜在冰鲜鸡肉保鲜中的应用[J].食品研究与开发, 2018,39(22):157-163. [15]袁晓菁,王灏,戴婷婷,等.壳聚糖复合纳米凝胶的聚合诱导自组装制备及生物应用[J].功能高分子学报,2018,31(4):340-349. [16]肖慧,董永星.改性壳聚糖处理微生物浸矿废水[J].化工环保, 2018, 38(5): 565-569. [17]邹倩,邱宝伟,梁雪,等.壳聚糖/大豆油/栀子黄可食性抗菌油墨的制备及性能测定[J].现代化工,2018,38(6):69-72. [18]赵雪,展义臻.蚕丝织物微波多功能环保改性工艺[J].丝绸, 2018,55(8): 12-16. [19]马准,李治学,王小濛, 等.改性壳聚糖离子交换膜制备研究进展[J].科学技术与工程,2018,18(18):131-139.[20]闫国卿.pH超敏感聚原酸酯药物载体的设计、制备及抗肿瘤活性研究[D].合肥:安徽大学,2017.[21]Guma SR,Lee DA,Gordon N,et al.Natural killer cell therapy and aerosol interleukin-2 for the treatment of osteosarcoma lung metastasis.Pediatr Blood Cancer.2014;61(4):618-626.[22]Taran SJ,Taran R,Malipatil NB.Pediatric Osteosarcoma: An Updated Review.Indian J Med Paediatr Oncol. 2017;38(1):33-43.[23]刘晓,邵方元,陈宏远.阿霉素抗肿瘤分子机制的研究进展[J].中国医药生物技术,2012,7(5):373-375.[24]Ayla S,Seckin I,Tanriverdi G,et al.Doxorubicin induced nephrotoxicity: protective effect of nicotinamide.Int J Cell Biol. 2011;2011:390238.[25]Cagel M,Grotz E,Bernabeu E,et al.Doxorubicin: nanotechnological overviews from bench to bedside.Drug Discov Today. 2017;22(2): 270-281.[26]李佳平,唐良萏.阿霉素诱导人卵巢癌细胞凋亡和周期特异性的实验研究[J].现代妇产科进展,2000, 9(5):338-340.[27]吉顺莉,张春燕,戈延茹.纳米载药系统的研究进展[J].中国药业, 2010, 19(14):82-83.[28]Zhang G,Zeng X,Li P.Nanomaterials in cancer-therapy drug delivery system. J Biomed Nanotechnol. 2013;9(5):741-750.[29]马鸿云,庄新明,考彦斌,等.控释纳米粒子在骨肿瘤化疗方面的应用[J].中国组织工程研究,2018,22(26):4247-4252.[30]周雅轩.纳米载药系统在医药领域中的应用进展[J].天津药学, 2012, 24(1):47-49.[31]李霏霏,张娜.纳米凝胶载体系统的研究进展[J].中国药学杂志, 2016, 51(3):177-182.[32]武敬亮,刘晨光,黄振华,等. pH值响应性透明质酸纳米粒的制备及作为阿霉素载体的研究[J].功能材料, 2012,43(12):1654-1657.[33]Dreher MR,Liu W,Michelich CR,et al.Tumor vascular permeability, accumulation, and penetration of macmmolecular drug carriers.Natl Cancer Inst.2006;98(5):335. [34]Nomura T,Koreeda N,Yamashita F,et al.Effect of particle size and charge Off the disposition of lipid carriers after intratu-moral injection into tissueisolated tumors.Pharm Res. 1998;15(1):128.[35]张丽娜,郭卫东.抗肿瘤药物引起的肝损伤[J].世界最新医学信息文摘, 2016,16(39):29-30.[36]张王宁,李爱平,刘少博,等.基于代谢组学的阿霉素肾病大鼠模型损伤程度评价[J].中草药, 2018,49(2):360-367.[37]Carvalho C,Santos RX,Cardoso S,et al.Doxorubicin:the good,the bad and the ugly effect.Curr Med Chem.2009;16(25):3267-3285.[38]郑梦颖.化疗药物对重要脏器不良反应的研究进展[J].重庆医学, 2012, 41(14):1431-1433. |