Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (1): 146-151.doi: 10.3969/j.issn.2095-4344.0425
Previous Articles Next Articles
Mesenchymal stem cells for diabetic foot: existing problems and better application
Xiang Jie, Zhang Jie
- Department of Endocrinology, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Revised:2017-10-16Online:2018-01-08Published:2018-01-08 -
Contact:Zhang Jie, Master, Associate chief physician, Master’s supervisor, Department of Endocrinology, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Xiang Jie, Studying for master’s degree, Department of Endocrinology, the First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:the Natural Science Foundation of Guangxi Zhuang Autonomous Region, No. 2013GXNSFAA278002
CLC Number:
Cite this article
Xiang Jie, Zhang Jie. Mesenchymal stem cells for diabetic foot: existing problems and better application[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(1): 146-151.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
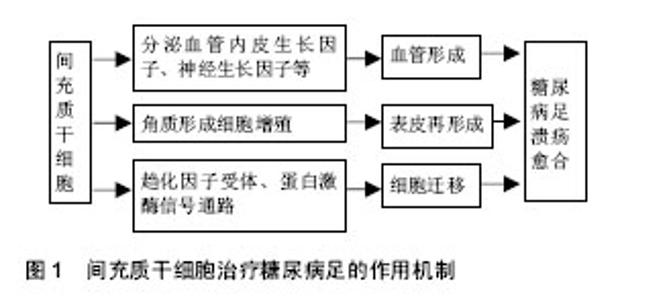
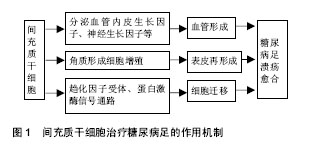
2.1 间充质干细胞简介 间充质干细胞是一种具有多向分化和自我更新能力的成体干细胞,20世纪60年代首次从骨髓中被鉴定。此后,间充质干细胞在多种组织中分离出来,包括骨髓、脐血、胎盘、脂肪、牙龈、口腔黏膜、羊水、脑等[9-10]。间充质干细胞可以向多种细胞分化,如成骨细胞、软骨细胞、脂肪细胞,成肌细胞、血管内皮细胞、神经细胞等。现有的技术可以很成熟的对其进行分离、扩增等体外培养,因此间充质干细胞可以作为组织工程研究中理想的种子细胞。间充质干细胞具有容易分离、增殖潜能高及基因稳定的优点,还能迁移到受损组织对抗炎症、影响微环境,促血管生成、抗纤维化、抗凋亡,释放细胞因子参与修复过程和组织再生[11]。间充质干细胞已被用来治疗很多缺血性疾病,例如缺血性心脑血管疾病,糖尿病足同样能得到改善,很多动物模型和临床试验都证实了间充质干细胞的作用,其治疗糖尿病足的主要机制在于:第一,旁分泌多种生长因子,如血管内皮生长因子等促进血管形成,第二,促 进角质形成细胞参与伤口表皮形成,调节局部微环境和免疫反应,第三,促进细胞向伤口组织迁移,增加肉芽组织的形成。上述机制协同作用加速糖尿病足伤口的修复[12],增加溃疡处的血供、促进肉芽组织形成、刺激表皮再生,增加保肢率[13-14]。目前临床研究尚未发现明显副作用或者病情恶化,也提示间充质干细胞治疗相对安全。下文对不同组织来源间充质干细胞治疗糖尿病足的作用机制和现有的临床研究进一步阐述(图1)。"
| [1] Ibrahim A. IDF Clinical practice recommendation on the diabetic foot: a guide for healthcare professionals. Diabetes Res Clin Pract. 2017;127:285-287.[2] 中华医学会糖尿病学分会.中国2型糖尿病防治指南(2013年版)[J].中华内分泌代谢杂志,2014,30(10):26-89.[3] Jiang Y, Ran X, Jia L, et al. Epidemiology of type 2 diabetic foot problems and predictive factors for amputation in China. Int J Low Extrem Wounds. 2015; 14(1):19-27.[4] Brod M, Nikolajsen A, Weatherall J, et al. The Economic Burden of Post-prandial Hyperglycemia (PPH) Among People with Type 1 and Type 2 Diabetes in Three Countries. Diabetes Ther. 2016;7(1):75-90.[5] 中国医疗保健国际交流促进会糖尿病足病分会.中国糖尿病足诊治指南[J].中华医学杂志,2017,97(4):251-258.[6] Zhao QS, Xia N, Zhao N, et al. Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. Int J Biol Sci. 2013;10(1):80-89. [7] Li S, Wang X, Li J, et al. Advances in the Treatment of Ischemic Diseases by Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:5896061.[8] Maxson S, Lopez EA, Yoo D, et al. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1(2):142-149.[9] Marfia G, Navone SE, Di Vito C, et al. Mesenchymal stem cells:potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis. 2015;11(4):183-206.[10] El-Demerdash RF, Hammad LN, Kamal MM, et al. A comparison of Wharton's jelly and cord blood as a source of mesenchymal stem cells for diabetes cell therapy. Regen Med. 2015;10(7):841-855.[11] Rani S, Ryan AE, Griffin MD, et al. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015; 23(5):812-823.[12] Marrotte EJ, Chen DD, Hakim JS, et al. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest. 2010;120(12):4207-4219.[13] Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:18104.[14] Schiavetta A, Maione C, Botti C, et al. A phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of the naples and pietra ligure evaluation of stem cells study. Stem Cells Transl Med. 2012; 1(7):572-578.[15] Hua J, Gong J, Meng H, et al. Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biol Int. 2013; 38(2):198-210.[16] Kato J, Kamiya H, Himeno T, et al. Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J Diabetes Complications. 2014;28(5):588-595.[17] Wan J, Xia L, Liang W, et al. Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J Diabetes Res. 2013;2013(9): 647107.[18] O'Loughlin A, Kulkarni M, Creane M, et al. Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes. 2013;62(7):2588-2594.[19] Hou C, Shen L, Huang Q, et al. The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials. 2013;34(1):112-120.[20] Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856-1868.[21] Xu J, Wu W, Zhang L, et al. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes. 2012;61(11):2906-2912.[22] Li M, Zhao Y, Hao H, et al. Mesenchymal stem cell-conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int J Low Extrem Wounds. 2015;14(1):73-86.[23] Iwamoto S, Lin X, Ramirez R, et al. Bone marrow cell mobilization by the systemic use of granulocyte colony-stimulating factor (GCSF) improves wound bed preparation. Int J Low Extrem Wounds. 2013;12(4):256-264.[24] Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986-18001.[25] You HJ, Namgoong S, Han SK, et al. Wound-healing potential of human umbilical cord blood-derived mesenchymal stromal cells in vitro-a pilot study. Cytotherapy. 2015;17(11):1506-1513.[26] Xia N, Xu JM, Zhao N, et al. Human mesenchymal stem cells improve the neurodegeneration of femoral nerve in a diabetic foot ulceration rats. Neurosci Lett. 2015;597:84-89.[27] Elsharawy MA, Naim M, Greish S. Human CD34+ stem cells promote healing of diabetic foot ulcers in rats.Interactive Cardiovascular and Thoracic Surgery. 2012;14(3):288-293.[28] Maharlooei MK, Bagheri M, Solhjou Z, et al. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract. 2011;93(2):228-234.[29] Kim SW, Zhang HZ, Guo L, et al. Amniotic Mesenchymal Stem Cells Enhance Wound Healing in Diabetic NOD/SCID Mice through High Angiogenic and Engraftment Capabilities. PLoS One. 2012;7(7):e41105.[30] Kuo YR, Wang CT, Cheng JT, et al. Adipose-derived Stem Cells Accelerate Diabetic Wound Healing through the Induction of Autocrine and Paracrine Effects. Cell Transplant. 2016;25(1):71-81.[31] Nie C, Yang D, Xu J, et al. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011; 20(2):205-216.[32] Kato Y, Iwata T, Morikawa S, et al. Allogeneic transplantation of an adipose-derived stem cell (ASC) sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes. 2015;64(8): 2723-2734.[33] Amos PJ, Kapur SK, Stapor PC, et al. Human Adipose-Derived Stromal Cells Accelerate Diabetic Wound Healing: Impact of Cell Formulation and Delivery. Tissue Eng Part A. 2010;16(5):1595-1606.[34] Abd-Allah SH, El-shal AS, Shalaby SM, et al. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life. 2015;67(9):701-709.[35] Kong P, Xie X, Li F, et al. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats.Biochem Biophys Res Commun. 2013;438(2):410-419.[36] Wang H, Chen L, Liu Y, et al. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation.Am J Transl Res. 2016; 8(11):4912-4921.[37] Skardal A, Mack D, Kapetanovic E, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. 2012;1(11):792-802.[38] Jun EK, Zhang Q, Yoon BS, et al. Hypoxic Conditioned Medium from Human Amniotic Fluid-Derived Mesenchymal Stem Cells Accelerates Skin Wound Healing through TGF-β/SMAD2 and PI3K/Akt Pathways. Int J Mol Sci. 2014;15(1):605-628. [39] Nowak WN, Borys S, Kusinska K, et al. Number of circulating pro-angiogenic cells, growth factor and anti-oxidative gene profiles might be altered in type 2 diabetes with and without diabetic foot syndrome. J Diabetes Investig. 2014;5(1):99-107.[40] Mizukami H, Yagihashi S. Exploring a New Therapy for Diabetic Polyneuropathy–The Application of Stem Cell Transplantation. Front Endocrinol. 2014;5:45.[41] Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11(2):189-197.[42] Liu N, Tian J, Jin C, et al. Migration of CXCR4 gene-modified bone marrow-derived mesenchymal stem cells to the acute kidney injury.J Cell Biochem. 2013;114(12):2677-2689.[43] Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92(1):26-36.[44] Yang SS, Kim NR, Park KB, et al. A phase I study of human cord blood-derived mesenchymal stem cell therapy in patients with peripheral arterial occlusive disease. Int J Stem Cells. 2013;6(1):37-44. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [5] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [6] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [7] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [8] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [9] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [10] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [11] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [12] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [13] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [14] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [15] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||