Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (1): 140-145.doi: 10.3969/j.issn.2095-4344.0424
Previous Articles Next Articles
Exosomes: a new strategy for mesenchymal stem cell transplantation in the treatment of osteoarthritis
Luo Xin, Yu Li-mei
- Key Laboratory of Cell Engineering in Guizhou Province, Basic and Clinical Innovation Team of Bone Marrow and Amniotic Stem Cells, Cell Engineering Center of Guizhou Province, Engineering Research Center of Stem Cells and Regeneration of Zunyi City, the Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China
-
Revised:2017-06-16Online:2018-01-08Published:2018-01-08 -
Contact:Yu Li-mei, M.D., Professor, Master’s supervisor, Key Laboratory of Cell Engineering in Guizhou Province, Basic and Clinical Innovation Team of Bone Marrow and Amniotic Stem Cells, Cell Engineering Center of Guizhou Province, Engineering Research Center of Stem Cells and Regeneration of Zunyi City, the Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
About author:Luo Xin, Master, Key Laboratory of Cell Engineering in Guizhou Province, Basic and Clinical Innovation Team of Bone Marrow and Amniotic Stem Cells, Cell Engineering Center of Guizhou Province, Engineering Research Center of Stem Cells and Regeneration of Zunyi City, the Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
Supported by:the National Natural Science Foundation of China, No. 81260507; Science and Technology Support Project in Zunyi, No. Zunshikehe (2016)6.
CLC Number:
Cite this article
Luo Xin, Yu Li-mei. Exosomes: a new strategy for mesenchymal stem cell transplantation in the treatment of osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(1): 140-145.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
2.1 关节软骨退行性变与骨性关节炎的发病机制 健康的关节软骨属透明软骨,表面光滑,呈淡蓝色,厚1-5 mm。关节软骨在关节活动中负责传导生物负载、吸收震荡,以及抗磨损和润滑作用。30岁以后,人的关节软骨趋于纤维变性,弹性和延伸能力弱化,加上关节液减少,使关节腔变得干燥,软骨易于磨损和损伤。随着年龄的增长,人体机能和运动能力逐渐下降,软骨下滋养血管数量减少,软骨生理、生化异常改变,细胞外基质蛋白多糖生物合成和分解异常,软骨细胞不能合成正常的具有长链结构的透明质酸和聚氨基葡萄糖,导致关节软骨局部软化、失去弹性及易于丢失基质,关节僵硬和炎性症状逐渐加重[4]。 软骨细胞中活性组分与基质等成分的合成与分解受到一系列分子的严格调控,而微环境的动态平衡则是保持关节软骨正常发挥功能的关键。骨形态发生蛋白和软骨源性形态发生蛋白在促进合成代谢方面发挥重要作用。其中骨形态发生蛋白3、骨形态发生蛋白7、软骨源性形态发生蛋白1和软骨源性形态发生蛋白2均在正常软骨组织中高表达,而在骨性关节炎损伤的软骨中低表达或不表达,并在体内外软骨形成实验中发挥促进作用。相反,白细胞介素17及其相关分子则负向调控骨形态发生蛋白和软骨源性形态发生蛋白的表达[5-6]。其中,白细胞介素17β在关节软骨的中区和深区表达,为主要负反馈作用分子,白细胞介素1和肿瘤坏死因子α等炎性因子也参与了关节软骨基质的降解。 骨性关节炎的病理机制中涉及NF-κB、p38MAPK和Wnt/β-catenin等细胞信号通路、巨噬细胞自噬清除、多种转录因子和miRNA的调控,以及肿瘤坏死因子α、白细胞介素1β、白细胞介素6、白细胞介素10等促炎与抑炎因子失衡、雌激素和机械应力等细胞外因素刺激等[7-8]。近年来有关外泌体在骨性关节炎发病机制中的作用不断被发现,早期关节腔微环境中的外泌体能代偿性分泌一些抑炎因子和miRNA等保护因素来延缓疾病进程;而中晚期,外泌体则会分泌过多的血管生成因子、促纤维化蛋白和促炎miRNA等来加速骨性关节炎的病理进展。 现有骨性关节炎的治疗主要包括非类固醇类抗炎药物、中医药及外科手术。1994年Brittberg等[9]首次报道了自体骨软骨移植术(马赛克成形术),但因软骨膜移植存在供区损伤等问题,难以适合较大面积的软骨缺损修复;随之发展的同种异体软骨移植术,也因移植物尺寸难控制,存在传染病风险和免疫排斥反应等,且有效作用期较短,成本较高,难以普及。自体软骨细胞移植技术,虽然治疗效果让人惊喜,但只适用于小面积软骨损伤,而且会遗留一部分损伤无法修复,或加重疾病进展[10]。21世纪以来,基于干细胞移植的细胞治疗已成为热点,动物实验和临床研究均证实了它的有效性,为骨性关节炎的治愈带来了新策略。 2.2 外泌体的特征与间充质干细胞的分泌功能 在囊泡转运过程中,依据转运位置的不同可分为早期内涵体、晚期内涵体和循环内涵体,其中晚期内涵体会形成多泡体,并能进一步分泌形成外泌体。1980年,这些小囊泡首次发现于羊网织红细胞的分泌物中[11]。1996年,Raposo等[12]发现B淋巴细胞分泌的外泌体具有激活免疫系统的作用,可参与适应性免疫应答。此后研究者们相继报道了外泌体囊泡中含有多种功能性蛋白,并不断阐明外泌体在细胞间的信号传递作用,即外泌体能够运载mRNA至靶细胞,翻译成相应的功能蛋白,还能负载miRNA,沉默某些功能基因表达。 随着外泌体在众多疾病中的病理作用被不断明确,2010年,间充质干细胞来源外泌体也被首次报道,其可明显减轻小鼠心肌缺血再灌注损伤[13],至此,外泌体所产生的强大作用日益受到学术界的高度关注。间充质干细胞来源外泌体不仅表达外泌体特异性表面标志物CD9和CD81,还表达间充质干细胞特异性表型CD29、CD44、CD73和CD90等[14]。 大多数间充质干细胞来源的外泌体除携带细胞膜骨架蛋白、微管蛋白、肌动蛋白和肌球蛋白外,还携带 CD9、CD63、CD81、CD82、Hspa8、Hsp60、Hsp70和Hsp90、ALG-2相互作用蛋白Ⅹ与肿瘤易感基因101蛋白,代谢相关蛋白GAPDH、LDHA、PGK1和PKM,以及组织/细胞类型特异性MHC-Ⅰ和MHC-Ⅱ等功能性蛋白[14]。间充质干细胞来源的外泌体还能转运mRNA、miRNA和siRNA等多种RNA,其中mRNA可直接在靶细胞中翻译出相应的功能蛋白质,发挥再生修复作用;miRNA 则通过沉默或抑制目标mRNA翻译来下调蛋白质在靶细胞中的表达(表1);siRNA则可敲除受体细胞中的目标基因达到基因沉默的作用[15]。外泌体也参与多种微环境中细胞信号转导,因此间充质干细胞来源外泌体在疾病发生、发展中的变化及疾病治疗中的作用日益受到关注。"

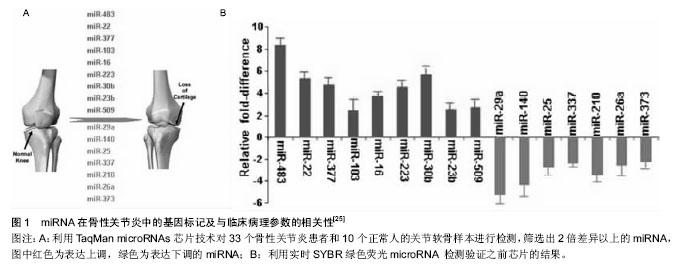
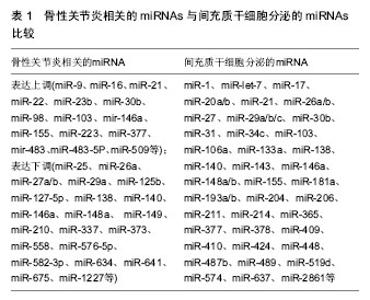
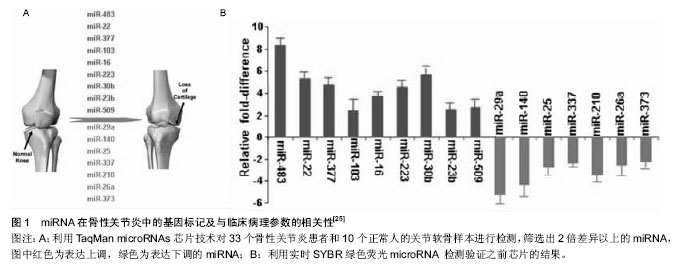
2.3 外泌体与骨性关节炎微环境变化 关节腔是一个密闭的低氧环境,软骨细胞能够在此进行正常新陈代谢。骨性关节炎患者关节腔的极低氧环境明显加剧了关节软骨细胞的分解代谢,其中低氧诱导因子是协调骨性关节炎极低氧环境最主要的转录因子,低氧诱导因子1α可诱导血管内皮生长因子大量合成,形成侵蚀性血管翳,进一步破坏关节软骨[16]。滑膜组织在低氧微环境中出现代偿性增生,趋化因子与其受体结合产生的趋化作用可诱导炎细胞浸润与促炎因子释放,形成慢性滑膜炎;同时软骨下骨供应血管功能紊乱进一步加剧低氧微环境的改变;恶性循环下,极低氧环境还能降低碱性磷酸酶的合成,促进白细胞介素1、白细胞介素6和MCP-1等促炎因子及趋化因子CXCL-12、COX-2、血管内皮生长因子和前列腺素E2等生成,诱发微环境中慢性炎症反应、软骨下骨硬化与骨赘形成[17]。研究证明,这些骨性关节炎病理微环境的改变均与局部外泌体所携带的多种蛋白和RNA转运密切相关[18]。 骨性关节炎微环境改变的另一个重要因素是胆固醇代谢。骨性关节炎发展中,受损软骨细胞的肝X受体α/β (Liver X Receptor α/β, LXR α/β)会大量流失,LXRα/β是胆固醇转运泵载脂蛋白A1(apolipoprotein A1, ApoA1)和ATP结合盒转运蛋白A1(ATP binding cassette subfamily A member 1, ABCA1)的关键转录调节因子,LXRα/β的减少会导致骨性关节炎微环境中ApoA1和ABCA1表达显著下调,从而阻碍胆固醇外流过程,导致细胞内脂质过度积累,进一步破坏软骨细胞[19]。外泌体广泛参与骨性关节炎微环境中的胆固醇代谢,其携带的转录因子胆固醇调节元件结合蛋白2(sterol regulatory element binding protein-2, SREBP-2)和miRNA-33a是胆固醇代谢的基本调节因子。Kostopoulou等[20]课题组在一系列关于骨性关节炎患者胆固醇微环境代谢的研究中发现,miRNA-33a在骨性关节炎患者的软骨细胞中显著升高,通过沉默靶标蛋白Smad7的转录过程提高转化生长因子β1的表达,再通过TGF-β1/PI3K/AKt/SREBP-2细胞信号途径大量增加软骨细胞内的胆固醇合成;miRNA-33a同时还能显著减少ABCA1和ApoA1的mRNA表达水平,从而阻碍胆固醇逆向转运过程,加重骨性关节炎的发展进程。Kostopoulou等[21]还发现,在骨性关节炎患者RNA中大量存在SREBP-2 G/C序列的高频突变,能导致转化生长因子β诱导蛋白聚糖合成抑制,也是骨性关节炎微环境中胆固醇代谢障碍的机制之一。 微环境中Zn2+代谢失衡也与骨性关节炎密切相关,外泌体分泌的miRNA-488在骨性关节炎中表达下调,并受转化生长因子β3和白细胞介素1β的调控,能够下调锌转运因子8(Zinc transporter 8, ZIP8),进而下调Zn2+敏感的金属调节转录因子1(Metal regulatory transcription factor 1, MTF-1)表达,导致MMP-3,MMP-13和ADAMTS-5高表达,可见miRNA-488/ZIP8/MTF1/ MMPs-ADAMTS5可能是骨性关节炎发病机制中至关重要的代谢调节轴线[22]。骨性关节炎发生发展过程中,外泌体分泌的miRNA-155会显著上调,导致自噬关键调节因子Ulk1、FoxO3、Atg14、Atg5、Atg3、Gabarapl1和Map1lc3等分泌合成同样被抑制,致使微环境中免疫细胞的自噬清除功能紊乱[23]。 2.4 外泌体释放的miRNAs介导骨性关节炎损伤的再生修复 在骨性关节炎的损伤与修复机制中,广泛存在于生物基因组中的非编码miRNA备受关注[24],大规模基因芯片技术分析发现34个与骨性关节炎发病和修复机制相关的miRNAs,其中14个miRNAs在骨性关节炎患者软骨中表达上调(miR-9, mir-16, miR-21, miR-22, mir-23b, mir-30b, miR-98, mir-103, mir-146a, mir-223, mir-377, mir-483, miR-483-5p和mir-509),20个miRNAs在骨性关节炎患者软骨中表达下调(miR-25, miR-26a, mir-27a, mir-27b, miR-29a, miR-125b, miR-127-5p, miR-140, miR-146, miR-148a, miR-149, miR-210, miR-337, miR-373, miR-558, miR-576-5p, mir-634, mir-641, miR-675和mir-1227)[25-26]。而不同来源间充质干细胞分泌的外泌体携带了不同含量的miRNAs,其表达正好能反向调节上述骨性关节炎患者软骨中miRNAs的改变,可能是间充质干细胞发挥再生修复作用的新机制(图1)。"
| [1] Hootman JM, Helmick CG, Barbour KE, et al. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015-2040. Arthritis Rheumatol. 2016;68(7): 1582-1587.[2] Katsha AM, Ohkouchi S, Xin H, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011; 19(1):196-203.[3] Camussi G, Deregibus MC, Bruno S, et al. Exosomes/ microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838-848.[4] Stufkens SA, Knupp M, Horisberger M, et al. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.[5] Wang K, Xu J, Cai J, et al. Serum levels of resistin and interleukin-17 are associated with increased cartilage defects and bone marrow lesions in patients with knee osteoarthritis. Mod Rheumatol. 2017;27(2):339-344.[6] van der Kraan PM, Berenbaum F, Blanco FJ, et al. Translation of clinical problems in osteoarthritis into pathophysiological research goals. RMD Open. 2016;2(1):e000224.[7] 王华敏,宓轶群,刚嘉鸿.信号通路在膝骨关节炎实验研究中的进展[J].中国组织工程研究,2016, 20(2):267-272.[8] Kato T, Miyaki S, Ishitobi H, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. [9] Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-895.[10] Viste A, Piperno M, Desmarchelier R, et al. Autologous chondrocyte implantation for traumatic full-thickness cartilage defects of the knee in 14 patients: 6-year functional outcomes. Orthop Traumatol Surg Res. 2012;98(7):737-743.[11] Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967-978.[12] Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996; 183(3):1161-1172.[13] Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214-222.[14] Pashoutan Sarvar D, Shamsasenjan K, Akbarzadehlaleh P. Mesenchymal Stem Cell-Derived Exosomes: New Opportunity in Cell-Free Therapy. Adv Pharm Bull. 2016;6(3):293-299.[15] Lässer C, Eldh M, Lötvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012;(59):e3037.[16] Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399-408.[17] Chenevier-Gobeaux C, Simonneau C, Lemarechal H, et al. Effect of hypoxia/reoxygenation on the cytokine-induced production of nitric oxide and superoxide anion in cultured osteoarthritic synoviocytes. Osteoarthritis Cartilage. 2013; 21(6):874-881.[18] Toh WS, Lai RC, Hui JHP, et al. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56-64.[19] Tsezou A, Iliopoulos D, Malizos KN, et al. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J Orthop Res. 2010;28(8):1033-1039.[20] Kostopoulou F, Malizos KN, Papathanasiou I, et al. MicroRNA-33a regulates cholesterol synthesis and cholesterol efflux-related genes in osteoarthritic chondrocytes. Arthritis Res Ther. 2015;17:42.[21] Kostopoulou F, Gkretsi V, Malizos KN, et al. Central role of SREBP-2 in the pathogenesis of osteoarthritis. PLoS One. 2012;7(5):e35753.[22] Song J, Kim D, Lee CH, et al. MicroRNA-488 regulates zinc transporter SLC39A8/ZIP8 during pathogenesis of osteoarthritis. J Biomed Sci. 2013;20:31.[23] D'Adamo S, Alvarez-Garcia O, Muramatsu Y, et al. MicroRNA-155 suppresses autophagy in chondrocytes by modulating expression of autophagy proteins. Osteoarthritis Cartilage. 2016;24(6):1082-1091.[24] Guo H, Ingolia NT, Weissman JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835-840.[25] Iliopoulos D, Malizos KN, Oikonomou P, et al. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3(11):e3740.[26] Tsezou A. Osteoarthritis year in review 2014: genetics and genomics. Osteoarthritis Cartilage. 2014;22(12):2017-2024.[27] Nakamura Y, Inloes JB, Katagiri T, et al. Chondrocyte-specific microRNA-140 regulates endochondral bone development and targets Dnpep to modulate bone morphogenetic protein signaling. Mol Cell Biol. 2011;31(14):3019-3028.[28] Miyaki S, Sato T, Inoue A, et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24(11):1173-1185.[29] Dudek KA, Lafont JE, Martinez-Sanchez A, et al. Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem. 2010;285(32): 24381-24387.[30] Vonk LA, Kragten AH, Dhert WJ, et al. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2014;22(1):145-153.[31] Akhtar N, Rasheed Z, Ramamurthy S, et al. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010; 62(5):1361-1371.[32] Matsukawa T, Sakai T, Yonezawa T, et al. MicroRNA-125b regulates the expression of aggrecanase-1 (ADAMTS-4) in human osteoarthritic chondrocytes. Arthritis Res Ther. 2013; 15(1):R28.[33] Park SJ, Cheon EJ, Lee MH, et al. MicroRNA-127-5p regulates matrix metalloproteinase 13 expression and interleukin-1β-induced catabolic effects in human chondrocytes. Arthritis Rheum. 2013;65(12):3141-3152.[34] Park SJ, Cheon EJ, Kim HA. MicroRNA-558 regulates the expression of cyclooxygenase-2 and IL-1β-induced catabolic effects in human articular chondrocytes. Osteoarthritis Cartilage. 2013;21(7):981-989.[35] Díaz-Prado S, Cicione C, Muiños-López E, et al. Characterization of microRNA expression profiles in normal and osteoarthritic human chondrocytes. BMC Musculoskelet Disord. 2012;13:144.[36] Li J, Huang J, Dai L, et al. miR-146a, an IL-1β responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis Res Ther. 2012;14(2):R75.[37] Zhang Y, Jia J, Yang S, et al. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp Mol Med. 2014;46:e79.[38] Jones SW, Watkins G, Le Good N, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17(4):464-472. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Peng Zhihao, Feng Zongquan, Zou Yonggen, Niu Guoqing, Wu Feng. Relationship of lower limb force line and the progression of lateral compartment arthritis after unicompartmental knee arthroplasty with mobile bearing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1368-1374. |
| [5] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [6] | Liu Xiangxiang, Huang Yunmei, Chen Wenlie, Lin Ruhui, Lu Xiaodong, Li Zuanfang, Xu Yaye, Huang Meiya, Li Xihai. Ultrastructural changes of the white zone cells of the meniscus in a rat model of early osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1237-1242. |
| [7] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [8] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [9] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [10] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [11] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [12] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [13] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [14] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [15] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||