Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (20): 5331-5340.doi: 10.12307/2026.150
Previous Articles Next Articles
Chitosan hydrogel drug delivery system is a safer and more effective solution for treating oral ulcers
Wang Jieyan, Yao Jiayi, Xin Yingtong, Zhang Xinwen, Li Riwang, Liu Dahai
- Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding, School of Animal Science and Technology, Foshan University, Foshan 528231, Guangdong Province, China
-
Accepted:2025-05-30Online:2026-07-18Published:2025-12-03 -
Contact:Li Riwang, Lecturer, Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding, School of Animal Science and Technology, Foshan University, Foshan 528231, Guangdong Province, China Liu Dahai, Professor, Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding, School of Animal Science and Technology, Foshan University, Foshan 528231, Guangdong Province, China -
About author:Wang Jieyan, Master candidate, Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding, School of Animal Science and Technology, Department of Medicine, Foshan University, Foshan 528231, Guangdong Province, China Yao Jiayi, Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding, School of Animal Science and Technology, Department of Medicine, Foshan University, Foshan 528231, Guangdong Province, China -
Supported by:2023 Guangdong Basic and Applied Basic Research Fund (Provincial and Municipal Joint Fund) Guangdong-Foshan Region Cultivation Project, No. 2023A1515140076 (to LRW); 2022 Guangdong Basic and Applied Basic Research Fund (Provincial and Municipal Joint Fund) Youth Project, No. 2022A1515110595 (to LRW); 2024 Medical Scientific Research Foundation of Guangdong Province, No. A2024290 (to LRW); National Natural Science Foundation of China, No. 82270413 (to LDH); Guangdong Natural Science Foundation, No. 2023A1515011581 (to LDH); Guangdong University Science and Technology Achievement Transformation Center Project, No. zc03040000560 (to LDH); Guangdong Provincial General Colleges and Universities Key Field Special Project (Biomedicine and Health), No. 2022ZDZX2057 (to LDH); Foshan University Student Academic Fund Project, No. xsjj202412zra01 (to LDH); Foshan University Student Academic Fund Project, No. xsjj202412zrb02 (to LRW)
CLC Number:
Cite this article
Wang Jieyan, Yao Jiayi, Xin Yingtong, Zhang Xinwen, Li Riwang, Liu Dahai. Chitosan hydrogel drug delivery system is a safer and more effective solution for treating oral ulcers[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5331-5340.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
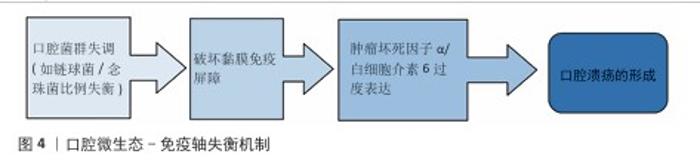
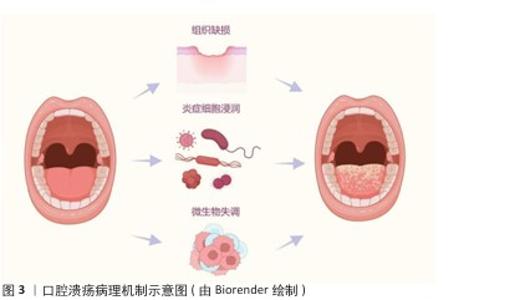
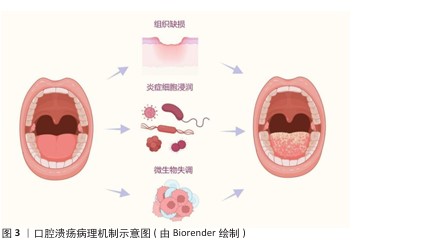
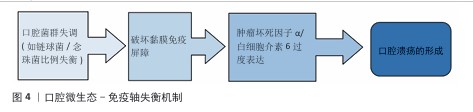
2.1.2 微生态失衡与免疫应答 口腔微生态系统由超过 700种微生物组成,其稳态维持依赖于共生菌(如唾液链球菌)与宿主免疫的动态平衡[17]。当口腔微生态系统失衡时,致病菌(如幽门螺杆菌、具核梭杆菌)过度增殖,通过释放脂多糖激活 Toll样受体通路,诱导巨噬细胞分泌肿瘤坏死因子α、白细胞介素6 等促炎因子,同时抑制调节性 T细胞功能,打破辅助性T细胞1/辅助性T细胞2免疫平衡[18],这种免疫紊乱进一步损伤黏膜屏障,形成“微生物感染 -炎症放大-组织损伤”的恶性循环(图4)。在复发性口腔溃疡患者口腔菌群中韦荣球菌属丰度显著升高,该菌代谢产物短链脂肪酸可通过激活NLRP3炎症小体加剧黏膜炎症。 2.1.3 临床治疗瓶颈分析 传统口腔溃疡治疗的局限见表2。喷雾剂、普通凝胶、含漱液等剂型在口腔动态环境中滞留时间短(≤2 h),药物利用率不足30%,并且缺乏微环境调控能力,细菌清除率仅40%-70%,远低于理想要求[18]。传统口腔溃疡治疗的局限主要体现在:①黏膜黏附性不足:市售凝胶(如地塞米松凝胶)依赖物理吸附,在唾液冲刷和咀嚼运动下滞留时间< 2 h,难以维持甲硝唑(最低抑菌浓度≥8 μg/mL)等药物的有效浓度,导致抗菌效果受限;②药物释放不可控:软膏剂的突释效应(如皮质类固醇初期浓度过高引发血糖紊乱)与缓释能力不足(无法持续调控慢性炎症核因子κB通路),形成“浓度波动-疗效不稳定”困境;③功能单一化:现有制剂多聚焦抗菌或抗炎(如氯己定含漱液仅清除50%-60%细菌,无促修复功能),缺乏对血管生成(血管内皮生长因子表达)、上皮再生(成纤维细胞迁移)的调控,导致愈合周期长达 7-14 d(年复发率> 50%),与理想体系的 “多效协同” 需求差距显著[19]。壳聚糖水凝胶通过化学改性、三维多孔网络及正电荷介导的细菌吸附,同时调控巨噬细胞极化(M1→M2)促进组织修复,完美适配“黏附-抗菌-修复”的理想治疗体系,为突破传统瓶颈提供材料学解决方案。 "
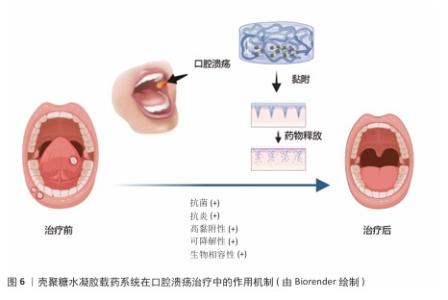
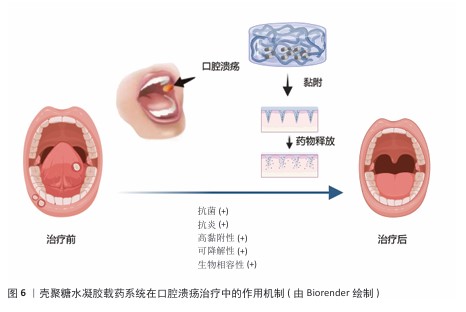
2.3 壳聚糖水凝胶的独特优势 2.3.1 壳聚糖水凝胶生物学特性与口腔环境适配性 口腔作为一个潮湿、动态并富含复杂微生物群落的特殊部位,对药物递送系统提出了较高的生物学要求。壳聚糖水凝胶作为一种天然阳离子多糖材料,展现出良好的生物相容性、生物可降解性及低毒性,可与口腔黏膜组织安全接触,避免引发免疫排斥或毒性反应,保障使用安全性。壳聚糖水凝胶可在口腔湿润环境中逐步降解,减少长期滞留带来的异物感和不良反应,体现出优越的可降解性[20]。 壳聚糖分子中的氨基在唾液中电离为阳离子,可与黏膜表面带负电的糖蛋白发生静电作用,从而增强局部附着力,延长药物在病灶区域的滞留时间,克服唾液冲刷、吞咽和言语活动等因素导致的药物丧失。壳聚糖水凝胶在温和条件下形成的三维水凝胶网络具有良好的柔韧性与机械强度,可紧密贴合黏膜表面并适应咀嚼与口腔运动所带来的形变,显著提升患者舒适度与依从性[21];此外,壳聚糖水凝胶具备天然的抗菌能力,能够通过破坏细菌细胞膜、螯合金属离子等机制有效抑制常见口腔病原体的生长,降低口腔溃疡继发感染风险。壳聚糖水凝胶优良的保湿性能亦有助于维持溃疡局部的湿润微环境,促进上皮细胞迁移与组织修复[22]。综合来看,壳聚糖水凝胶凭借高度适配口腔环境的多重生物学特性,成为构建理想口腔药物递送系统的重要基础材料。 2.3.2 壳聚糖水凝胶载药系统在口腔溃疡治疗中所需要的性能 在口腔溃疡治疗过程中,壳聚糖水凝胶载药系统需要具备一系列关键性能,以确保它能够有效促进溃疡愈合、减少感染风险并提升患者舒适度。壳聚糖水凝胶系统必须具备良好的湿附着力,以便水凝胶能够紧密贴合口腔黏膜,确保药物在病变部位的持续释放,这种湿附着力主要来源于水凝胶在水性介质中的溶胀能力以及它与黏膜表面的氢键结合[23];同时水凝胶需要保持较低的吸水率,以防止因过度膨胀而导致黏附性能下降。通过调整水凝胶的基质成分、搭载药物类型以及官能团结构,可以有效优化水凝胶的黏附性能。 药物释放周期的精准调控对于治疗效果至关重要。理想的水凝胶载药系统应能够实现药物的持续和受控释放,避免药物的快速释放或过早耗尽。药物释放机制主要受侵蚀、溶胀、扩散和降解4个因素的影响[24]。通过优化水凝胶的交联方式和基质成分可以调节其降解周期,从而满足长期药物作用的需求:化学交联方式虽然更稳定,但该交联方式制备水凝胶的降解速度较慢;而物理交联方式更安全,并且交联制备的水凝胶降解速度相对较快。此外,pH值、温度等外部条件也会对药物释放产生影响[25]。 抗菌抗炎性能是壳聚糖水凝胶载药系统的另一大优势。在口腔溃疡愈合过程中,感染和炎症是常见的并发症,因此水凝胶需要具备良好的抗菌性能,以防止细菌感染并促进伤口愈合[26]。壳聚糖本身具有天然的抗菌特性,其多孔结构可以作为防止细菌侵入的屏障。此外,通过搭载抗菌药物和抗炎药物,可以进一步增强水凝胶的抗菌和抗炎能力,促进伤口愈合[27]。 系统的安全性是不容忽视的。理想的水凝胶载药系统必须具备良好的生物相容性和无毒性。天然生物聚合物在酶降解后产生的副产物通常对生物体具有良好的耐受性,不会引发毒性反应。相比之下,合成聚合物在制备过程中可能会残留小分子物质,这些残留物可能会引起生物毒性或免疫排斥反应[28]。在水凝胶制备过程中,物理交联方式由于不使用有机溶剂或引发剂,因此更加安全[29]。由此可见,壳聚糖水凝胶载药系统在口腔溃疡治疗中需要具备良好的湿附着力、精准的药物释放周期、强大的抗菌抗炎性能以及卓越的安全性,这些性能的综合优化将显著提升口腔溃疡的治疗效果,缩短愈合时间,并减轻患者痛苦[30]。 壳聚糖水凝胶载药系统在口腔溃疡治疗中的作用机制,见图6。 "
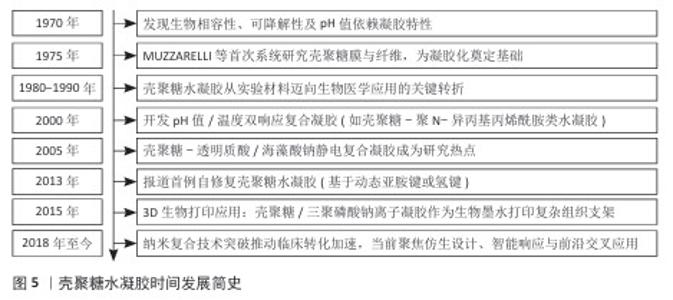
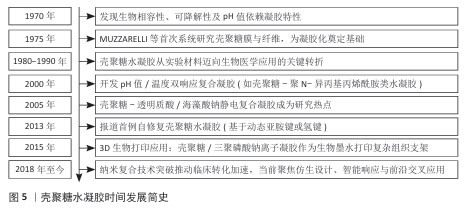
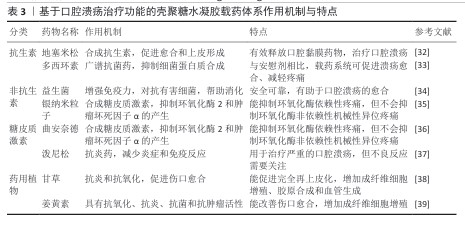
2.4 壳聚糖水凝胶载药系统的研究进展 2.4.1 基于治疗功能的水凝胶分类与制备方法 目前应用于口腔溃疡治疗的壳聚糖水凝胶载药系统,依据所负载药物的功能和来源可分为四大类:抗生素类抗菌剂、非抗生素类抗菌剂、皮质类固醇类药物以及天然药物类[31]。传统治疗中常用皮质类固醇和抗生素控制炎症与感染,但长期使用可能引发高血糖、血压紊乱、耐药性等不良反应,促使新型替代药物的开发不断推进,涵盖抗菌、抗炎、抗氧化等作用机制。不同类型药物对水凝胶的结构设计、负载方式及释放机制具有不同要求[32]。壳聚糖作为天然阳离子多糖,可通过物理或化学交联与其他高分子(如海藻酸钠、透明质酸)协同构建多孔三维网络,以增强水凝胶的稳定性、强度及药物负载效率,常见的制备策略包括离子交联(如 Ca2+或 Fe3+)、冷冻-融解法、pH值或温度响应性溶胶-凝胶转化等。其中,近年来发展出的巯基化壳聚糖、多巴胺改性壳聚糖水凝胶在智能黏附与高效递送方面展现出良好潜力[33]。 抗生素类抗菌剂常用于治疗口腔溃疡相关的细菌感染,地塞米松和多西环素是代表性药物。地塞米松作为广谱合成抗生素具有促愈合与上皮化作用,通过明胶-聚多巴胺-纳米黏土水凝胶递送地塞米松可以提升靶向性与生物利用度[34]。多西环素为四环素类衍生物,抑菌机制明确,可减少病变期疼痛并加速愈合过程。利用壳聚糖构建的抗生素水凝胶系统能在局部实现缓释,减少系统毒副作用与耐药风险[35]。 非抗生素类抗菌剂包括金属类材料(如银纳米颗粒)、益生菌等。银纳米粒子具备广谱高效的抗菌活性,虽作用机制尚未完全明晰,但在伤口敷料中应用广泛[36]。益生菌通过调节菌群稳态和增强宿主免疫在局部发挥促愈合效果,例如益生菌-海藻酸钙/岩藻多糖水凝胶可显著促进溃疡愈合[34]。 皮质类固醇如曲安奈德和泼尼松,主要通过抑制环氧合酶2和肿瘤坏死因子α介导的炎症通路缓解疼痛和黏膜炎反应[37]。水凝胶作为皮质类固醇载体,既能缓释药效,又减少了传统软膏剂型在唾液环境中易被稀释的问题,然而类固醇长期使用需谨慎,因它可能带来多种代谢紊乱[38]。 天然药用植物因作用靶点多、不良反应低而备受关注。甘草提取物富含多种抗炎、抗氧化次生代谢产物,能增强成纤维细胞增殖和血管生成,已在不同形式(贴片、漱口水、水凝胶)中成功应用。姜黄素亦通过抑制氧化应激和促进胶原合成等途径加速上皮再生与组织修复,在动物实验中的修复效果良好[39]。 表3总结了不同类别药物在口腔溃疡治疗中的应用,涵盖抗生素、非抗生素、糖皮质激素和药用植物等类别,详细比较了各类药物的作用机制与特性,为临床治疗提供了参考。 2.4.2 壳聚糖水凝胶的载药机制与药物释放特性 壳聚糖水凝胶的载药机制主要依托其独特的物理化学结构与动态响应能力,通过物理包裹、化学键合及环境响应型释放实现药物的高效负载与可控递送[40]。壳聚糖水凝胶的三维多孔网络结构(孔径50-200 nm)可通过氢键、静电相互作用或疏水作用力物理包裹药物分子;同时,壳聚糖分子链上的氨基与羟基可参与共价交联或离子交联形成稳定的载药网络,通过调节交联密度精准控制药物包封率[41]。在药物释放特性上,壳聚糖水凝胶表现出三重控释机制:①扩散主导型释放:药物通过浓度梯度从溶胀的凝胶基质中扩散,释放速率受壳聚糖分子质量及羧甲基化修饰调控;②溶蚀/降解驱动型释放:水凝胶在口腔溶菌酶或弱酸性环境中逐步降解,释放药物并伴随凝胶体积收缩,降解速率与壳聚糖脱乙酰度呈负相关;③智能响应型释放:通过引入pH值敏感基团或温度敏感组分实现溃疡微环境(pH=4.5-5.5)触发释药或体温诱导凝胶相变,例如pH值敏感型壳聚糖/海藻酸钠复合水凝胶在酸性条件下质子化氨基增加,网络松弛加速药物释放,而中性环境下则通过静电交联抑制突释效应[42]。研究显示,壳聚糖水凝胶的释药动力学符合扩散控制模型或非费克扩散模型,突释期(0-2 h)释放量可控制在 15%-25%,后续24 h内维持零级释放(累积释药率70%-90%);此外,壳聚糖通过唾液黏蛋白的硫醇基与壳聚糖氨基结合,可延长水凝胶在溃疡面的滞留时间(≥ 6 h),减少唾液冲刷导致的药物损失,同时该水凝胶阳离子特性可破坏细菌细胞膜(对金黄色葡萄球菌抑菌率> 90%)并中和致炎因子(如白细胞介素6、肿瘤坏死因子α),形成“载药-缓释-抗菌-促愈”四位一体的协同治疗模式[43]。LIU等[33]研究发现,与传统庆大霉素膜相比,含槲皮素的壳聚糖复合膜通过缓释抗氧化成分可显著缩小溃疡面积,而负载四环素的柠檬酸功能化水凝胶通过pH值依赖性释药使局部药物浓度维持在最低抑菌浓度以上长达12 h。未来,通过分子设计或复合纳米颗粒可进一步优化壳聚糖水凝胶载药系统的机械强度与生物功能性。 2.4.3 壳聚糖水凝胶载药系统的促修复分子机制 壳聚糖水凝胶通过多维度分子调控网络实现促修复作用,涵盖炎症微环境重塑、细胞迁移/增殖驱动及信号通路靶向干预三大核心机制[44]。在炎症调控方面,NGUYEN等[45]研究发现,壳聚糖/银掺杂生物活性玻璃水凝胶通过失活核因子κB 通路降低炎性牙髓干细胞中白细胞介素1β、白细胞介素6 水平,同时激活磷脂酰肌醇-3-激酶/蛋白激酶B通路促进抗炎型M2巨噬细胞极化,进而调控免疫微环境。在细胞迁移层面,壳聚糖通过整合素-黏着斑激酶信号轴增强成纤维细胞迁移能力,并激活Wnt/β-catenin 信号通路促进上皮细胞增殖。WANG等[46]研究发现,载益母草碱壳聚糖复合水凝胶通过调控磷脂酰肌醇-3-激酶/蛋白激酶B通路使牙周膜干细胞内碱性磷酸酶活性和矿化结节形成量提高。此外,壳聚糖的电荷特性可中和溃疡面带负电的坏死组织碎片,通过转化生长因子β/Smad 信号通路上调胶原合成,增强血管内皮生长因子分泌(血管密度提升50%)以改善局部血供[47]。值得注意的是,壳聚糖的分子质量和脱乙酰度直接影响其促愈效能:低分子质量壳聚糖(25-50 kD)通过激活Toll 样受体4/MyD88通路增强巨噬细胞趋化,而高脱乙酰度(≥85%)壳聚糖通过静电作用捕获血小板衍生生长因子BB,促进牙槽骨再生[48]。然而,当前研究对壳聚糖调控 Notch通路(影响细胞命运决定)和 Hippo通路(调节组织再生规模)的作用仍缺乏系统解析,需结合单细胞测序和基因编辑筛选技术进一步揭示其多靶点协同机制。"
| [1] SENEL S, OZDOGAN AI, AKCA G. Current status and future of delivery systems for prevention and treatment of infections in the oral cavity. Drug Deliv Transl Res. 2021;11(4):1703-1734. [2] JAVIER F, EGIDO-MORENO S, SCHEMEL-SUAREZ M, et al. Treatment of recurrent aphtous stomatitis: a systematic review. Med Oral Patol Oral Cir Bucal. 2023;28(1): E87-E98. [3] SHAHRIN E, NARUDIN N, SHAHRI N, et al. A comparative study of adsorption behavior of rifampicin, streptomycin, and ibuprofen contaminants from aqueous solutions onto chitosan: dynamic interactions, kinetics, diffusions, and mechanisms. Emerg Contam. 2023;9(1):2509-2529. [4] LIN D, YANG L, WEN L, et al. Crosstalk between the oral microbiota, mucosal immunity, and the epithelial barrier regulates oral mucosal disease pathogenesis. Mucosal Immunol. 2021;14(6):1247-1258. [5] MOUSSA SG, HOFFY NM, MOUSELHY YY, et al. Sustainable treatment of oral traumatic ulcers with licorice containing hydrogels: integrating computational modeling, quality by design, green synthesis, and molecular biological evaluation. Pharmaceutics. 2023; 15(12):1708-1736. [6] GASMI-BENAHMED A, NOOR S, MENZEL A, et al. Oral aphthous: pathophysiology, clinical aspects and medical treatment. Arch Razi Inst. 2021;76(5):1155-1163. [7] GHORBANI A, AKBARI J, BOORBOOR M, et al. Evaluation of zinc sulfate mucoadhesive formulation on recurrent aphthous stomatitis: a randomized double-blind, placebo-controlled clinical trial. Bmc Oral Healt. 2020;20(1):121. [8] ARABPOUR Z, ABEDI F, SALEHI M, et al. Hydrogel-Based skin regeneration. Int J Mol Sci. 2024;25(4):1982. [9] 曾欣,刘帆.水凝胶载药系统在牙周炎治疗中的研究进展[J].四川大学学报(医学版),2023,54(4):721-725. [10] FEDERER C, KURPIERS M, BERNKOP-SCHNURCH A. Thiolated chitosans: a multi-talented class of polymers for various Applications. Biomacromolecules. 2021;22(1):24-56. [11] WANG Y, ZOU J, CAI M, et al. Applicatoin of chitosan-based hydrogel in oral tissue engineering. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023;48(1):138-147. [12] SONG F, KONG Y, SHAO C, et al. Chitosan-based multifunctional flexible hemostatic bio-hydrogel. Acta Biomater. 2021;136: 170-183. [13] GUO S, REN Y, CHANG R, et al. Injectable self-healing adhesive chitosan hydrogel with antioxidative, antibacterial, and hemostatic activities for rapid hemostasis and skin wound healing. ACS Appl Mater Interfaces. 2022;14(30):34455-34469. [14] TIAN S, DING T, LI H. Oral microbiome in human health and diseases. mLife. 2024; 3(3):367-383. [15] CUI C, MEI L, WANG D, et al. A self-stabilized and water-responsive deliverable coenzyme-based polymer binary elastomer adhesive patch for treating oral ulcer. Nat Commun. 2023;14(1):7707. [16] BLOCH S, HAGER-MAIR FF, ANDRUKHOV O, et al. Oral streptococci: modulators of health and disease. Front Cell. 2024;14: 1357631. [17] EBHODAGHE SO. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. Biomater Sci Polym Ed. 2022;33(12):1595-1622. [18] YU Y, YANG D, LIN B, et al. Readily available oral prebiotic protein reactive oxygen species nanoscavengers for synergistic therapy of inflammation and fibrosis in inflammatory bowel disease. Acs Nano. 2024;18(21):13583-13598. [19] ZHOU Y, WANG M, YAN C, et al. Advances in the application of electrospun drug-loaded nanofibers in the treatment of Oral ulcers. Biomolecules. 2022;12(9):1804456. [20] ALMOHAMMED HI, KHALAF AK, ALBALAWI AE, et al. Chitosan-Based nanomaterials as valuable sources of anti-leishmanial agents: a systematic review. Nanomaterials. 2021;11(3):689. [21] NANIWA M, NAKATOMI C, HITOMI S, et al. Analgesic mechanisms of steroid ointment against oral ulcerative mucositis in a rat model. Inter J Mol Sci. 2021;22(22):12600. [22] DALESSANDRI D, ZOTTI F, LAFFRANCHI L, et al. Treatment of recurrent aphthous stomatitis (RAS; aphthae; canker sores) with a barrier forming mouth rinse or topical gel formulation containing hyaluronic acid: a retrospective clinical study. Bmc Oral Health. 2019;19:58. [23] GUAN T, LI J, CHEN C, et al. Self-Assembling peptide-based hydrogels for wound tissue repair. Adv Sci. 2022;9(10):456. [24] KASBAJI M, MENNANI M, OUBENALI M, et al. Bio-based functionalized adsorptive polymers for sustainable water decontamination: A systematic review of challenges and real-world implementation. Environ Pollut. 2023;335:122349. [25] SENEL S, IKINCI G, KAS S, et al. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm. 2000;193(2):197-203. [26] PIACENTINI M, BORGHETTI R, ZANCANARO DE FIGUEIREDO A, et al. Doxycycline: an option in the treatment of ulcerated oral lesions? J Clin Pharm Ther. 2019;44(6): 838-843. [27] NIMBENI S, NIMBENI B, DIVAKAR D. Role of chitosan in remineralization of enamel and dentin: a systematic review. Int J Clin Pediatr Dent. 2021;14(4):562-568. [28] CHENG H, HUANG Y, ZHU Z, et al. Adsorption of cadmium vapor by calcium-based adsorbents in simulated flue gas: Experimental and density functional theory studies. Appl Surf Sci. 2023;614:660. [29] KHAN M, STOJANOVIC G, REHMAN R, et al. Graphene oxide-functionalized bacterial cellulose-gelatin hydrogel with curcumin release and kinetics: in vitro biological evaluation. Acs Omega. 2023;8(43): 40024-40035. [30] ZHANG Y, HUANG Y. Rational design of smart hydrogels for biomedical applications. Front Chem. 2021;8:615665. [31] ZHANG W, BAO B, JIANG F, et al. Promoting oral mucosal wound healing with a hydrogel adhesive based on a phototriggered s-nitrosylation coupling reaction. Adv Mater. 2021;33(48):e2105667. [32] WANG Y, PAN Z, CUI J, et al. Adhesive hydrogel releases protocatechualdehyde-Fe 3+complex to promote three healing stages for accelerated therapy of oral ulcers. Acta Biomater. 2024;178:68-82. [33] LIU Y, REN S, JI H, et al. Study on the inhibition of inflammation by the cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathway and the promotion of wound healing of oral ulcer of Yangyin Shengji powder after chemotherapy. Ann Palliat Med. 2021;10(12):12716-12726. [34] AGHBASHLO M, AMIRI H, BASRI SMM, et al. Tuning chitosan’s chemical structure for enhanced biological functions. Trends Biotechnol. 2023;41(6):785-797. [35] LIU Z, LIU X, HAN Y, et al. Efficacy and safety of total glucosides of paeony in the treatment of recurrent aphthous ulcers: a systematic review and meta-analysis. Front Pharmacol. 2024;15:1378782. [36] HAN Z, YUAN M, LIU L, et al. pH-Responsive wound dressings: advances and prospects. Nanoscale Horiz. 2023; 8(4):422-440. [37] AN H, GU Z, ZHOU L, et al. Janus mucosal dressing with a tough and adhesive hydrogel based on synergistic effects of gelatin, polydopamine, and nano-clay. Acta Biomater. 2022;149:126-138. [38] KHAN S, SHETTY B, FAZAL I, et al. Licorice as a herbal extract in periodontal therapy. Drug Target Insights. 2023;17:70-77. [39] SAMIRANINEZHAD N, ASADI K, REZAZADEH H, et al. Using chitosan, hyaluronic acid, alginate, and gelatin-based smart biological hydrogels for drug delivery in oral mucosal lesions: A review. Int J Biol Macromol. 2023;252:126573. [40] KHAN F, ATIF M, HASEEN M, et al. Synthesis, classification and properties of hydrogels: their applications in drug delivery and agriculture. J Mater Chem B. 2022;10(2):170-203. [41] NASROLLAHZADEH M, SAJJADI M, IRAVANI S, et al. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohydr Polym. 2021;251:116986. [42] MATZEU G, NAVEH G, AGARWAL S, et al. Functionalized mouth-conformable interfaces for pH evaluation of the oral cavity. Adv Sci. 2021;8(12):e2203416. [43] DOU X, LI G, WANG S, et al. Probiotic-loaded calcium alginate/fucoidan hydrogels for promoting oral ulcer healing. Inter J Biol Macromol. 2023;244:125273. [44] TAGHIZADEH F, MEHRYAB F, MORTAZAVI S, et al. Thiolated chitosan hydrogel-embedded niosomes: a promising crocin delivery system toward the management of aphthous stomatitis. Carbohydr Polym. 2023;318:e2003416. [45] NGUYEN S, HIORTH M. Advanced drug delivery systems for local treatment of the oral cavity. Ther Deliv. 2015;6(5):595-608. [46] WANG W, XUE C, MAO X. Chitosan: structural modification, biological activity and application. Inter J Biolo Macromole. 2020;164:4532-4546. [47] ZHAO Y, RAN B, XIE X, et al. Developments on the smart hydrogel-based drug delivery system for oral tumor therapy. Gels. 2022; 8(11):478. [48] PAN Z, ZHANG X, XIE W, et al. Revisited and innovative perspectives of oral ulcer: from biological specificity to local treatment. Front Bioeng Biotechnol. 2024;12:412. [49] HASHTRODYLAR Y, RABBANI S, DADASHZADEH S, et al. Berberine-phospholipid nanoaggregate-embedded thiolated chitosan hydrogel for aphthous stomatitis treatment. Nanomedicine (Lond). 2023;18(19):1227-1246. [50] MILANDA T, CINDANA R, MOHAMMED AFA, et al. Alginate/Chitosan-based hydrogel film containing α-mangostin for recurrent aphthous stomatitis therapy in rats. Pharmaceutics. 2022;14(8):467. [51] NETSOMBOON K, JALIL A, LAFFLEUR F, et al. Thiolated chitosans: are cys-cys ligands key to the next generation? Carbohydr Polym. 2020;242:116395. [52] SU K, LI J, WU X, et al. One-Step synthesis of hydrogel adhesive with acid-responsive tannin release for diabetic oral mucosa defects healing. Adv Health Mater. 2024; 13(9):e2303252. [53] CATOIRA M, FUSARO L, DI FRANCESCO D, et al. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med. 2019;30(10):115. [54] GU R, ZHOU H, ZHANG Z, et al. Research progress related to thermosensitive hydrogel dressings in wound healing: a review. Nanoscale adv. 2023;5(22): 6017-6037. [55] CHEN J, REN J, WU Y, et al. Wet adhesive hydrogels based on niobium carbide for experimental research of oral mucosal impairment. Rsc Adv. 2024;14(18): 12935-12946. [56] REZAZADEH M, JAFARI N, AKBARI V, et al. A mucoadhesive thermosensitive hydrogel containing erythropoietin as a potential treatment in oral mucositis: in vitro and in vivo studies. Drug Deliv Transl Res. 2018; 8(5):1226-1237. [57] AL-MAWERI S, ALAIZARI N, ALHARBI A, et al. Efficacy of curcumin for recurrent aphthous stomatitis: a systematic review. J DermatolTreat. 2022;33(3):1225-1230. [58] BASSO F, SOARES D, PANSANI T, et al. Proliferation, Migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge. Lasers in Surg Med. 2016;48(10): 1006-1014. [59] ZHANG S, JIANG H, HUANG S, et al. Curdlan sulfate/<i>O</i>-linked quaternized chitosan nanoparticles acting as potential adjuvants promote multiple arms of immune responses. Carbohydr Polym. 2019;213: 100-111. [60] ZHANG X, WEI P, YANG Z, et al. Current progress and outlook of nano-based hydrogel dressings for wound healing. Pharmaceutics. 2023;15(1):68. [61] CHEN Z, WANG L, GUO C, et al. Vascularized polypeptide hydrogel modulates macrophage polarization for wound healing. Acta Biomater. 2023;155:218-234. [62] MONTAZERIAN H, BAIDYA A, HAGHNIAZ R, et al. Stretchable and bioadhesive gelatin methacryloyl-based hydrogels enabled by in situ dopamine polymerization. ACS Appl Mater Interfaces. 2021;13(34): 40290-40301. [63] CHI J, ZHANG X, CHEN C, et al. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact Mater. 2020;5(2):253-259. [64] WIECKIEWICZ M, BOENING K, GRYCHOWSKA N, et al. Clinical application of chitosan in dental specialities. Mini Rev Med Chem. 2017;17(5):401-409. [65] YI Z, LUO X, ZHAO L. Research advances in chitosan oligosaccharides: from multiple biological activities to clinical applications. Curr Med Chem. 2020;27(30):5037-5055. [66] SIGNORINI L, MARENZI G, FACENTE A, et al. Critical overview on pure chitosan-based scaffolds for bone tissue engineering: clinical insights in dentistry. Int J Med Sci. 2023;20(12):1527-1534. |
| [1] | Chen Ying, Sun Xuheng, Liu Qing, Xiao Cong, Jiang Hongjing, Lin Zhanyi. A serum-free culture medium for the early-stage formation of tissue-engineered vascular grafts [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5093-5102. |
| [2] | Cao Yuqing, Guo Meiling, Liu Feng, Wei Junchao. Preparation, classification and application of polysaccharide-based hydrogels in skin damage repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5257-5269. |
| [3] | Diao Youlu, Gao Jia, Pan Guoqing. Recruitable tissue repair biomaterials: advantages of regulating cell and factor migration and improving tissue integration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5270-5281. |
| [4] | He Zhenzhen, Huang Hanji, Wang Jiawei, Xie Qingtiao, Jiang Xianfang. Role of bioscaffolds in the repair of inflammation-driven bone and cartilage destruction and structural damage in temporomandibular joint [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5312-5320. |
| [5] | Xu Yixuan, Yao Jun, Liu Xulu, Li Xinlian, Liu Zhixiong, Zhang Zhihong. Vancomycin-containing porcine skin acellular extracellular matrix hydrogel promotes wound healing in skin infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5214-5228. |
| [6] | Bai Xiangyu, Huo Feng, Hao Yan, Wang Zecheng, Guo Xiaoyu. Platelet-derived growth factor BB-loaded chitosan/reduced graphene oxide scaffold for repairing alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 329-337. |
| [7] | Liu Xiaohong, Zhao Tian, Mu Yunping, Feng Wenjin, Lyu Cunsheng, Zhang Zhiyong, Zhao Zijian, Li Fanghong. Acellular dermal matrix hydrogel promotes skin wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 395-403. |
| [8] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [9] | Li Liang, Yang Han, Suo Hairui, Guan Lu, Wang Zhenlin. 3D printed methacrylated gelatin/chitosan scaffolds: evaluation of antibacterial, mechanical properties and cytocompatibility [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3636-3642. |
| [10] | Jia Wei, Zhang Mandong, Chen Weiyi, Wang Chenyan, Guo Yuan. Effects of femoral prosthetic materials on artificial knee arthroplasty performance [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1477-1481. |
| [11] | Wang Qian, Li Lu, Shu Jingyuan, Dong Zhiheng, Jin Youshi, Wang Qingshan. Micro-morphology and phase of zirconia-based nano-hydroxyapatite functional gradient biomaterials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1517-1521. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||