Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (14): 3636-3642.doi: 10.12307/2025.586
Previous Articles Next Articles
3D printed methacrylated gelatin/chitosan scaffolds: evaluation of antibacterial, mechanical properties and cytocompatibility
Li Liang1, Yang Han2, Suo Hairui2, Guan Lu1, Wang Zhenlin1
- 1No. 906 Hospital of People’s Liberation Army, Ningbo 315040, Zhejiang Province, China; 2School of Automation, Hangzhou Dianzi University, Hangzhou 310018, Zhejiang Province, China
-
Received:2024-12-20Accepted:2025-02-20Online:2026-05-18Published:2025-09-11 -
Contact:Wang Zhenlin, Associate chief physician, No. 906 Hospital of People’s Liberation Army, Ningbo 315040, Zhejiang Province, China -
About author:Li Liang, MD, Associate chief physician, No. 906 Hospital of People’s Liberation Army, Ningbo 315040, Zhejiang Province, China -
Supported by:2021 Ningbo Natural Science Foundation Project, No. 2021J238 (to LL)
CLC Number:
Cite this article
Li Liang, Yang Han, Suo Hairui, Guan Lu, Wang Zhenlin. 3D printed methacrylated gelatin/chitosan scaffolds: evaluation of antibacterial, mechanical properties and cytocompatibility[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3636-3642.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
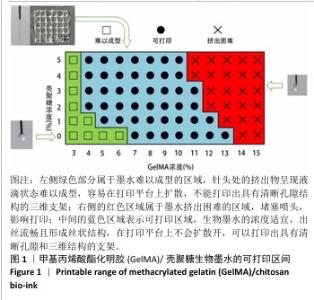
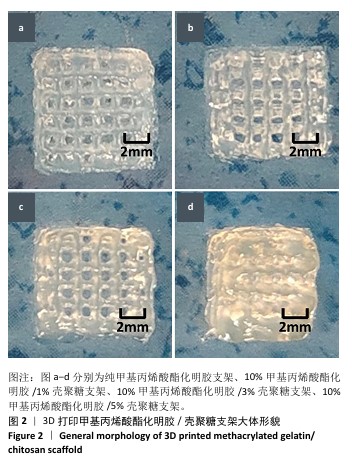
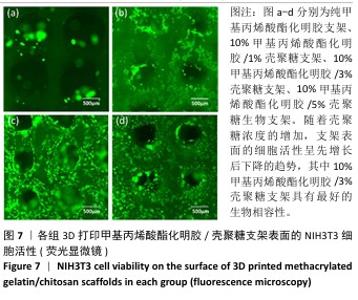
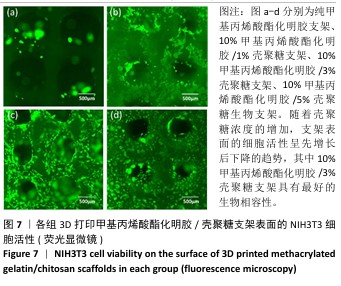
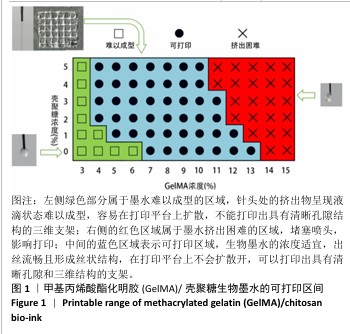
2.1 生物墨水的可打印性能测试结果 甲基丙烯酸酯化明胶/壳聚糖生物墨水在室温下的可打印区间通过判断挤出状态来确定。在图1中,左侧绿色部分属于墨水难以成型的区域,针头处的挤出物呈现液滴状态难以成型,容易在打印平台上扩散,不能打印出具有清晰孔隙结构的三维支架;右侧的红色区域属于墨水挤出困难的区域,堵塞喷头,影响打印;中间的蓝色区域表示可打印区域,生物墨水的浓度适宜,出丝流畅且形成丝状结构,在打印平台上不会扩散开,可以打印出具有清晰孔隙和三维结构的支架。从图1中可以看出,在室温下,纯甲基丙烯酸酯化明胶的打印区间在7%-13%之间,壳聚糖的加入可以使甲基丙烯酸酯化明胶的打印区间向低浓度扩展,最低可至4%;同时,向高浓度的甲基丙烯酸酯化明胶中加入壳聚糖会使其黏度增加而挤出困难。事实上,在打印区间的边界,虽然墨水可以挤出,但打印效果往往较差。 最终将10%甲基丙烯酸酯化明胶分别与0%,1%,3%和5%壳聚糖混合作为生物墨水,分别命名为G10、G10C1、G10C3、G10C5,进行3D打印(打印参数同上),见图2。"
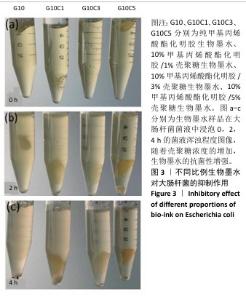
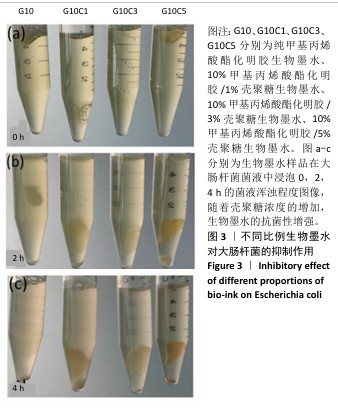
2.2 生物墨水的抗菌性检测结果 通过观察管内大肠杆菌的含量来判断不同浓度壳聚糖生物墨水的抗菌性,如图3所示。离心管内的培养基越浑浊,表明含有的大肠杆菌数量越多。实验开始时,4支离心管中的培养基浑浊程度接近一致,管内清澈,表明大肠杆菌较少,如图3a所示。置于37 ℃恒温培养2 h后,离心管内发生变化,其中G10组离心管内培养基变得最浑浊,有最多的大肠杆菌,G10C1组次之,随着壳聚糖浓度的增加,G10C3组离心管内培养基略微浑浊,而G10C5组相比于其他组离心管内的培养基更为清澈,证明有最少的大肠杆菌,如图3b所示。培养4 h后,各组离心管的培养基浑浊程度与2 h的结果基本保持一致,如图3c所示。结果说明随着壳聚糖浓度增加,生物墨水的抗菌性增强。"
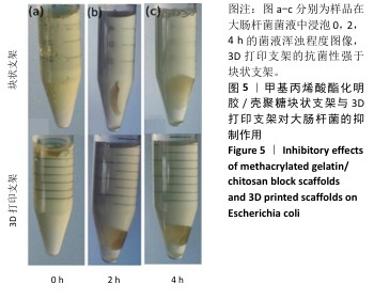
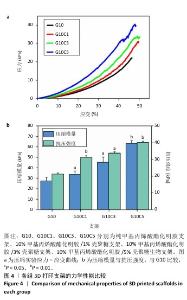
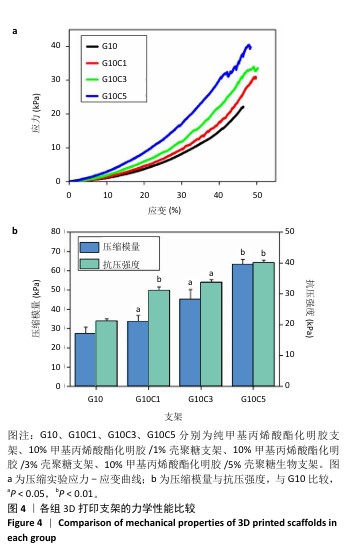
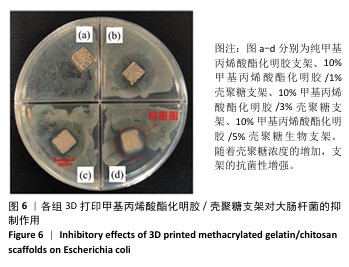
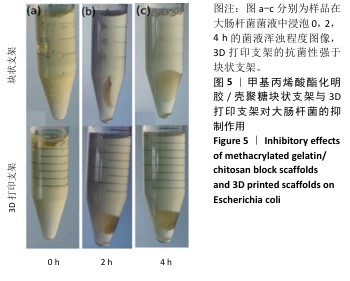
2.4 支架的抗菌性能测试结果 为了验证打印后支架的抗菌能力,观察相同浓度、质量下G10C3块状支架和3D打印支架所在离心管中培养基的浑浊程度,见图5。随着时间的增加,含有3D打印支架离心管内的培养基更加清澈,证明3D打印支架的抗菌性优于块状支架。3D打印支架由于具有更大的比表面积,与大肠杆菌接触的面积更大,因此具有比块状支架更强的抗菌性。 图6展示了4种3D打印支架的抗菌性能。可以看出,G10支架没有抗菌能力,而含壳聚糖支架各组形成了不同范围的抑菌圈,G10C1支架的抑菌圈直径为11.8 mm,随着壳聚糖浓度的增大,G10C3支架的抑菌圈达到了14.2 mm,而G10C5支架的抑菌圈则达到了17.3 mm。"
| [1] RUSSO A, CONCIA E, CRISTINI F, et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect. 2016;22:S27-S36. [2] ZERVOS M J, FREEMAN K, VO L, et al. Epidemiology and outcomes of complicated skin and soft tissue infections in hospitalized patients. J Clin Microbiol. 2012; 50(2):238-245. [3] MOHITI-ASLI M, POURDEYHIMI B, LOBOA EG. Novel, silver-ion-releasing nanofibrous scaffolds exhibit excellent antibacterial efficacy without the use of silver nanoparticles. Acta Biomater. 2014;10(5):2096-2104. [4] DUTTA P, RINKI K, DUTTA J. Chitosan: A Promising Biomaterial for Tissue Engineering Scaffolds. Springer Berlin Heidelberg. 2011:45-79. [5] AHMED S, ANNU, ALI A, et al. A review on chitosan centred scaffolds and their applications in tissue engineering. Int J Biol Macromol. 2018;116:849-862. [6] AHMADI F, OVEISI Z, SAMANI SM, et al. Chitosan based hydrogels: characteristics and pharmaceutical applications. Res Pharm Sci. 2015;10(1):1-16. [7] VENKATESAN J, KIM SK. Chitosan composites for bone tissue engineering--an overview. Mar Drugs. 2010;8(8):2252-2266. [8] ARCHANA D, DUTTA J, DUTTA P. Chitosan-pectin-titanium dioxide nano-composite film: An investigation for wound healing applications. Asian Chitin J. 2010;6:45-46. [9] USMAN A, ZIA KM, ZUBER M, et al. Chitin and chitosan based polyurethanes: A review of recent advances and prospective biomedical applications. Int J Biol Macromol. 2016;86:630-645. [10] WU J, LEI J, CHEN M, et al. Synthesis and Characterization of Photo-Cross-Linkable Silk Fibroin Methacryloyl Hydrogel for Biomedical Applications. ACS Omega. 2023;8(34):30888-30897. [11] GAO J, LI M, CHENG J, et al. 3D-Printed GelMA/PEGDA/F127DA Scaffolds for Bone Regeneration. J Funct Biomater. 2023;14(2):96. [12] YUE K, TRUJILLO-DE Santiago G, ALVAREZ MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254-271. [13] YING G, JIANG N, YU C, et al. Three-dimensional bioprinting of gelatin methacryloyl (GelMA). Biodes Manuf. 2018;1(4):215-224. [14] COLOSI C, SHIN SR, MANOHARAN V, et al. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater. 2016;28(4):677-684. [15] CHEN J, HUANG D, WANG L, et al. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J Colloid Interface Sci. 2020;574:162-173. [16] MEHDIKHANI M, YILGR P, POURSAMAR SA, et al. A hybrid 3d-printed and electrospun bilayer pharmaceutical membrane based on polycaprolactone/chitosan/polyvinyl alcohol for wound healing applications. Int J Biol Macromol. 2024;282:136692. [17] GUO W, MAO Y, TANG X, et al. Copper-Loaded Microporous Chitosan Generated within a 3D-Printed Polylactic Acid-Pearl Scaffold: Structure and Performance. ACS Appl Polym Mater. 2024;10:2541. [18] PEPPAS NA, BURES P, LEOBANDUNG W, et al. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27-46. [19] HOFFMAN AS. Hydrogels for biomedical applications. Ann N Y Acad Sci. 2001;944: 62-73. [20] 陈向标.水凝胶医用敷料的研究概况[J].轻纺工业与技术,2011, 40(1):66-68. [21] BERAM FM, ALI SN, MESBAHIAN G, et al. 3D Printing of Alginate/Chitosan-Based Scaffold Empowered by Tyrosol-Loaded Niosome for Wound Healing Applications: In Vitro and In Vivo Performances. ACS Appl Bio Mater. 2024;7(3):1449-1468. [22] LIU, LI W, LIU H, et al. Preparation of 3D Printed Chitosan/Polyvinyl Alcohol Double Network Hydrogel Scaffolds. Macromol Biosci. 2021; 21:2000398. [23] NEGI A, VERMA A, GARG M, et al. Osteogenic citric acid linked chitosan coating of 3D-printed PLA scaffolds for preventing implant-associated infections. Int J Biol Macromol. 2024;282:136968. [24] TEJAL VP, HEXIU J, SAYAN DD, et al. Zn@TA assisted dual cross-linked 3D printable glycol grafted chitosan hydrogels for robust antibiofilm and wound healing. Carbohydr Polym. 2024;344:122522. [25] ISHIHARA M, NAKANISHI K, ONO K, et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials. 2002;23(3):833-840. [26] 季冬青,孙丹丹,和焕香,等.壳聚糖抗菌活性研究进展[J].辽宁中医药大学学报,2018,20(3):82-85. [27] SU Y, LIU Y, HU X, et al. Caffeic acid-grafted chitosan/sodium alginate/nanoclay-based multifunctional 3D-printed hybrid scaffolds for local drug release therapy after breast cancer surgery. Carbohydr Polym. 2024;324:121441. [28] HUI I, PASQUIER E, SOLBERG A, et al. Biocomposites containing poly(lactic acid) and chitosan for 3D printing–Assessment of mechanical, antibacterial and in vitro biodegradability properties. J Mech Behav Biomed. 2023;147:106136. [29] ARTEMIS P, IOANNA K, ANNA M, et al. Optimization of chitosan-gelatin-based 3D-printed scaffolds for tissue engineering and drug delivery applications. Int J Pharmaceut. 2024;666:124776. [30] KOUMENTAKOU I, NOORDAM MJ, MICHOPOULOU A, et al. 3D-Printed Chitosan-Based Hydrogels Loaded with Levofloxacin for Tissue Engineering Applications. Biomacromolecules. 2023;24(9):4019-4032. [31] CUI H, CAI J, HE H, et al. Tailored chitosan/glycerol micropatterned composite dressings by 3D printing for improved wound healing. Int J Biol Macromol. 2024;255:127952. [32] SHAHROUDI S, PARVINNASAB A, SALAHINEJAD E, et al. Efficacy of 3D-printed chitosancerium oxide dressings coated with vancomycin-loaded alginate for chronic wounds management. Carbohydr Polym. 2024;349:123036. [33] LI S, XU Y, ZHENG L. High Self-Supporting Chitosan-Based Hydrogel Ink for InSitu 3D Printed Diabetic Wound Dressing. Adv Funct Mater. 2024;1:241625. [34] ZHANG W, SHI K, YANG J, et al. 3D printing of recombinant collagen/chitosan methacrylate/nanoclay hydrogels loaded with Kartogenin nanoparticles for cartilage regeneration. Regener Biomater. 2024; 11:97. [35] CH S, MISHRA P, PADAGA SG, et al. 3D‐Printed Inherently Antibacterial Contact Lens‐Like Patches Carrying Antimicrobial Peptide Payload for Treating Bacterial Keratitis. Macromal Biosci. 2024;24(4):2300418. [36] PAHLEVANZADEH F, EMADI R, KHARAZIHA M, et al. Amorphous magnesium phosphate-graphene oxide nano particles laden 3D-printed chitosan scaffolds with enhanced osteogenic potential and antibacterial properties. Biomater Adv. 2024;158:213760. [37] LIU Z, SHI J, CHEN L, et al. 3D printing of fish-scale derived hydroxyapatite/chitosan/PCL scaffold for bone tissue engineering. Int J Biol Macromol. 2024; 274(2):13172. [38] KOUMENTAKOU I, NOORDAM MJ, MICHOPOULOU A, et al. 3D-Printed Chitosan-Based Hydrogels Loaded with Levofloxacin for Tissue Engineering Applications. Biomacromolecules. 2023;24(9): 4019-4032. [39] VASEGHI A, SADEGHIZADEH M, HERB M, et al. 3D Printing of Biocompatible and Antibacterial Silica-Silk-Chitosan-Based Hybrid Aerogel Scaffolds Loaded with Propolis. ACS Appl Bio Mater. 2024; 20:697. [40] SUO H, ZHANG D, YIN J, et al. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater Sci Eng C. 2018;92:612-620. [41] ZHAO X, LIU Y, JIA P, et al. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res Ther. 2021;12(1):196. [42] CROISIER F, JÉRÔME C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49(4):780-792. [43] PÉREZ-DÍAZ M, ALVARADO-GOMEZ E, MAGAÑA-AQUINO M, et al. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater Sci Eng C. 2016;60:317-323. [44] FREIER T, KOH HS, KAZAZIAN K, et al. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26(29):5872-5878. |
| [1] | Sun Lei, Zhang Qi, Zhang Yu. Pro-osteoblastic effect of chlorogenic acid protein microsphere/polycaprolactone electrospinning membrane [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1877-1884. |
| [2] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| [3] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [4] | Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970. |
| [5] | Bai Xiangyu, Huo Feng, Hao Yan, Wang Zecheng, Guo Xiaoyu. Platelet-derived growth factor BB-loaded chitosan/reduced graphene oxide scaffold for repairing alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 329-337. |
| [6] | Zhou Shibo, Yu Xing, Chen Hailong, Xiong Yang. Nanocrystalline collagen-based bone combined with Bushen Zhuangjin Decoction repairs bone defects in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 354-361. |
| [7] | Liu Xiaohong, Zhao Tian, Mu Yunping, Feng Wenjin, Lyu Cunsheng, Zhang Zhiyong, Zhao Zijian, Li Fanghong. Acellular dermal matrix hydrogel promotes skin wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 395-403. |
| [8] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [9] | Wang Yu, Fan Minjie, Zheng Pengfei. Application of multistimuli-responsive hydrogels in bone damage repair: special responsiveness and diverse functions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 469-479. |
| [10] | . Effect of mussel-derived antimicrobial peptide-coated modified prosthesis on prevention of early periprosthetic joint infection and regulation of bone transfer [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 278-287. |
| [11] | Guo Jingwen, Wang Qingwei, He Zijun, Hu Zihang, Chen Zhi, Zhu Rong, Wang Yuming, Liu Wenfei, Luo Qinglu. Intra-articular injection of different concentrations of silicon-based bioceramics in treatment of knee osteoarthritis in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 288-295. |
| [12] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| [13] | Wu Tianyi, Miao Yiming, Wan Kaichen, Teng Yun, Zou Jun. Protective effect of mesoporous ZLN005@polydopamine nanoparticles on chondrocytes in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3576-3585. |
| [14] | Wang Hao, He Qin, Wang Pingxi, Zhang Jun, Wu Zhilin. Deferoxamine-loaded strontium alginate hydrogel promotes the repair of skull injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3609-3617. |
| [15] | Shi Xiaonan, Wu Xuan, Zhang Daxu, Hu Jingjing, Zheng Yazhe, Liu Yutong, Zhao Shuo, Li Weilong, Ye Shujun, Wang Jingyi, Yan Li. Preparation and characterization of 3D printed microstructured silk fibroin scaffold for liver injury repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3618-3625. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||