Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (20): 5312-5320.doi: 10.12307/2026.669
Previous Articles Next Articles
Role of bioscaffolds in the repair of inflammation-driven bone and cartilage destruction and structural damage in temporomandibular joint
He Zhenzhen1, Huang Hanji2, Wang Jiawei3, Xie Qingtiao1, Jiang Xianfang1
- 1Guangxi Key Laboratory of Oral and Maxillofacial Rehabilitation and Reconstruction, Guangxi Clinical Research Center for Craniofacial Deformity, College & Hospital of Stomatology, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; 2Guangxi Engineering Center in Biomedical Materials for Tissue and Organ Regeneration, Collaborative Innovation Centre of Regenerative Medicine and Medical BioResource Development and Application, Guangxi Key Laboratory of Regenerative Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; 3Center for Reproduction and Genetics, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China
-
Accepted:2025-05-24Online:2026-07-18Published:2025-12-02 -
Contact:Jiang Xianfang, MD, Chief physician, Master’s supervisor, Guangxi Key Laboratory of Oral and Maxillofacial Rehabilitation and Reconstruction, Guangxi Clinical Research Center for Craniofacial Deformity, College & Hospital of Stomatology, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China Xie Qingtiao, MD, Associate chief physician, Master’s supervisor, Guangxi Key Laboratory of Oral and Maxillofacial Rehabilitation and Reconstruction, Guangxi Clinical Research Center for Craniofacial Deformity, College & Hospital of Stomatology, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:He Zhenzhen, Master candidate, Guangxi Key Laboratory of Oral and Maxillofacial Rehabilitation and Reconstruction, Guangxi Clinical Research Center for Craniofacial Deformity, College & Hospital of Stomatology, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:National Natural Science Foundation of China, No. 82160188 (to JXF); Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation, No. 2025GXNSFAA069065 (to XQT); Basic Research Capacity Improvement Program for Young and Middle-aged Teachers in Guangxi Universities, No. 2022KY0080 (to XQT)
CLC Number:
Cite this article
He Zhenzhen, Huang Hanji, Wang Jiawei, Xie Qingtiao, Jiang Xianfang. Role of bioscaffolds in the repair of inflammation-driven bone and cartilage destruction and structural damage in temporomandibular joint[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5312-5320.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

2.1 生物支架用于颞下颌关节炎骨与软骨修复研究的时间脉络 见表1。 19世纪初,科学家开始使用合成材料用于骨修复。1960年之前,颞下颌关节骨关节炎移植物为自体移植物,1960-1970年,学者们提出使用异种移植物作为关节假体。1982年,SONNENBURG等[11]使用同种异体材料完全置换颞下颌关节。1987年,美国材料专家LANGER和LANGER等[12]提出了组织工程概念,开启了细胞-材料结合使用时代。1994年,PUELACHER等[13]首次使用形状类似颞下颌关节盘支架与细胞结合,将该复合物植入裸鼠体内对新生软骨进行评估。2001年,SPRINGER等[14]研究颞下颌关节衍生细胞在不可吸收支架材料上的黏附、扩散和细胞外基质合成,材料在关节盘移植中能提供持久的结构支撑。2007年,SMITH 等[15]使用基于图像的设计和计算机软件,创建出用于骨组织再生的支架。2008年,LUMPKINS等[16]将异种支架用于颞下颌关节骨关节炎修复,使用3种常见的脱细胞方案形成脱细胞基质,其中十二烷基硫酸钠处理的猪关节盘与天然组织的能量耗散能力和抗变形能力相似。2014年,WU等[17]使用混合支架结合颞下颌软骨细胞修复颞下颌关节椎间盘穿孔。2021年,WANG等[18]将水凝胶和双相磷酸钙陶瓷结合,可促进干细胞增殖、成骨分化和软骨形成特异性基质分泌。2025年,JEONG等[19]使用配备空间控制生物活性的3D支架生物工程方法来进行软硬组织修复。 "

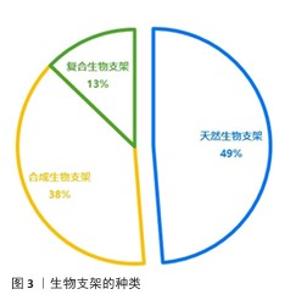
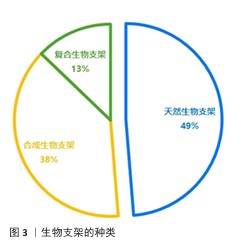
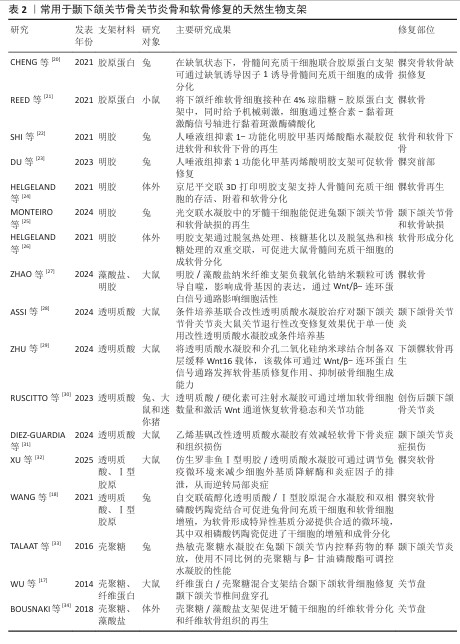
2.2.1 用于修复颞下颌关节骨关节炎的天然生物支架 常见的天然生物支架材料包括胶原蛋白、明胶、海藻酸钠和壳聚糖等,这些材料具有良好的生物相容性、可降解性和无毒性,但稳定性较差、机械强度较低且来源相对有限(表2)。天然支架材料可以通过物理或化学结合形成性能良好的水凝胶,例如壳聚糖/海藻酸钠复合物和明胶/海藻酸钠复合物。 胶原蛋白:颞下颌关节软骨主要由纤维软骨构成,基质以Ⅰ型胶原蛋白为主。由于源自自然且有良好的生物降解性和显著的抗拉强度,胶原蛋白成为组织工程中首选的生物相容性材料。与由巯基修饰的透明质酸与Ⅰ型胶原蛋白构成的水凝胶共培养时,软骨细胞展现出卓越的增殖能力以及优异的生物特性和耐受性[18],这种复合材料不仅有效缓解了传统胶原蛋白水凝胶常见的降解与收缩问题,还降低了材料的刚性和能量耗散性能。 明胶:作为部分降解的胶原蛋白,其化学成分与胶原蛋白密切相关。甲基丙烯酸明胶水凝胶因高生物相容性、生物可降解性和较低的成本而备受关注[22]。在明胶基质中,髁软骨和骨细胞均表现出良好的再生潜能,并能促进骨矿化[35]。由罗非鱼Ⅰ型明胶和透明质酸/硫酸软骨素构建的仿生双网络水凝胶,在组成、结构和机械强度方面均表现出优异的仿生性能,该水凝胶能够改善关节局部炎症微环境、促进颞下颌关节椎间盘细胞增殖、诱导滑膜间充质干细胞向纤维软骨分化,快速有效地修复大鼠颞下颌关节炎和小型猪颞下颌关节椎间盘穿孔缺陷[7]。 透明质酸:是细胞外基质的重要组成部分,能够模拟细胞外基质的特性并增强其周围微环境[36]。由于显著的保水性和膨胀能力,透明质酸有助于改善关节组织的结构和渗透性。通过在透明质酸中加入各种胶原或明胶可以进一步提升水凝胶的功能。将仿生罗非鱼Ⅰ型明胶与透明质酸结合不仅可以减少细胞外基质降解酶和炎症因子的产生,从而减轻颞下颌关节局部炎症,还可以通过调节巨噬细胞和T细胞的功能创造有利于修复的微环境[32]。 壳聚糖:由几丁质去乙酰化而成,能够抑制关节内的粘连形成并促进软骨修复[37]。透明质酸等细胞外基质化合物修饰的壳聚糖支架可以模拟软骨组织的微环境,为软骨再生创造适宜条件。然而,壳聚糖本身在诱导软骨生成方面的能力有限,单独作为软骨再生材料的修复效果并不理想,需要与其他材料复合使用。在壳聚糖/透明质酸水凝胶中培养脂肪组织来源间充质干细胞,并注入来自人类关节软骨细胞的细胞外囊泡,可以显著提高软骨形成关键基因SOX9和Ⅱ型胶原蛋白的表达、促进Ⅱ型胶原蛋白的产生,这一过程营造了理想的微环境,极大地促进了间充质干细胞向软骨细胞的分化,从而有效支持了软骨的修复与再生[38]。改变壳聚糖与β-甘油磷酸酯的比例并加入透明质酸,会显著影响水凝胶的凝胶时间、机械性能、膨胀能力和物理化学特性[33]。此外,丝素/壳聚糖水凝胶联合颞下颌关节来源的间充质干细胞可有效修复颞下颌关节椎间盘穿孔,增加软骨细胞外基质沉积,增强修复能力[17]。 海藻酸盐:是一种天然的、具有生物相容性和可生物降解的聚合物。在海藻酸钠三维培养体系中培养4周后,髁突软骨细胞表现出优异的分化能力[39]。壳聚糖/海藻酸盐复合结构能够增强牙髓干细胞向纤维软骨分化的潜力,促进纤维软骨组织的生成,可作为天然颞下颌关节盘的替代材料[34]。研究表明,壳聚糖/海藻酸盐水凝胶制作的仿生关节盘中心区域的存储模量低于天然颞下颌关节关节盘,而外周区域的存储模量与天然关节盘相当 [40]。 "
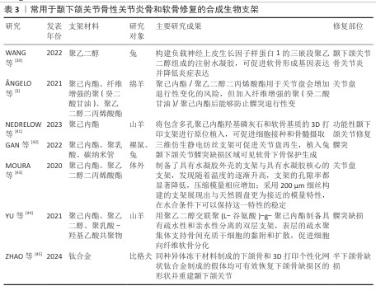
2.2.2 用于颞下颌关节骨性关节炎修复的合成支架 合成生物支架主要由各种聚合物组成,包括聚乙烯醇、聚己内酯和聚乙二醇二丙烯酸酯等,它们的优点是稳定性好、结构强度高、可降解性低、可批量生产,缺点是在合成过程中可能会释放单体和有毒物质,构成一定的生物安全性问题,见表3。 聚己内酯:是一种通过ε-己内酯的开环聚合反应制得的线型脂肪族聚酯,具有优越的生物降解性和生物相容性,成为组织再生应用中的理想材料。聚己内酯支架的刚度甚至高于天然关节软骨和椎间盘。聚己内酯/聚氨酯复合支架的力学特性与人类颞下颌关节盘的中心区域相似,而单纯聚氨酯支架的力学特性则更接近关节盘外部区域[46]。 聚乙二醇二丙烯酸酯:聚乙二醇二丙烯酸酯支架能够促进细胞黏附并提供润滑功能。当聚乙二醇二丙烯酸酯与聚己内酯等材料结合使用时,支架性能显著提升,因为聚己内酯可以提供优异的力学性能,而聚乙二醇二丙烯酸酯能为细胞分布和黏附创造理想的微环境[47]。将聚乙二醇二丙烯酸酯以壳核结构形式加入到聚己内酯水凝胶中形成多材料复合水凝胶,发现随着温度升高,支架的孔隙率减小,压缩模量增大[43];支架核心部分能够承受长时间的实质性应力,同时可复制天然椎间盘固有的生物力学特征;壳体部分可以模拟颞下颌关节的表面特征,通过应力分散机制减少摩擦,降低应力集中[3,43]。 聚乙烯醇:是一种可溶于水的合成聚合物,具有网状结构,但缺乏必要的机械强度[48]。聚乙烯醇支架具有良好的吸水能力、生物相容性和易加工性,是替代关节软骨和骨的优良材料,有望用于替代颞下颌关节椎间盘。与绵羊颞下颌关节椎间盘相比,25%聚乙烯醇水凝胶的拉伸破坏强度明显更高[49]。经冻融退火工艺制备的改性聚乙烯醇仿生水凝胶表现出抗膨胀能力、高机械强度和良好的生物相容性,其中退火聚乙烯醇为提供机械支撑的主要框架,而加入纳米片改性阴离子聚氨酯链的水凝胶则具备保水性和润滑功能,表现出低摩擦系数,在真实负载场景下该水凝胶能够有效分配施加的应力并吸收能量,表现出与天然颞下颌关节盘非常相似的行为[50]。触变超分子网络和聚合物合成的仿生双网络水凝胶展现出独特的剪切响应和润滑性能,该水凝胶由聚丙烯酰胺和聚乙烯醇组成的双网络框架支撑,具有较高的力学性能,同时超分子框架在剪切应力作用下的分解赋予了水凝胶优越的润滑性能[51]。为了提高力学性能,研究人员用聚己内酯植入物增强聚乙烯醇水凝胶的性能,聚乙烯醇/聚己内酯支架具有足够的机械强度,可以承受压缩和拉伸力,比天然关节盘具有更小的蠕变,并表现出适当的黏弹性,在循环压应力后能够恢复其初始最大强度,甚至超过山羊天然颞下颌关节关节盘,表明它能够承受异常咬合所造成的外力损伤[52]。 "
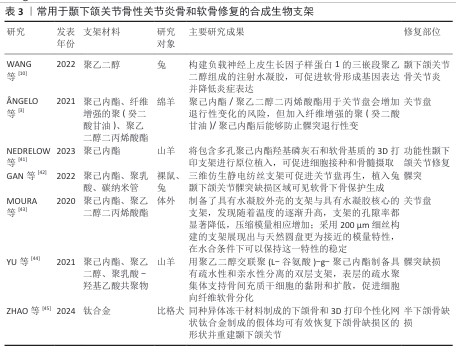
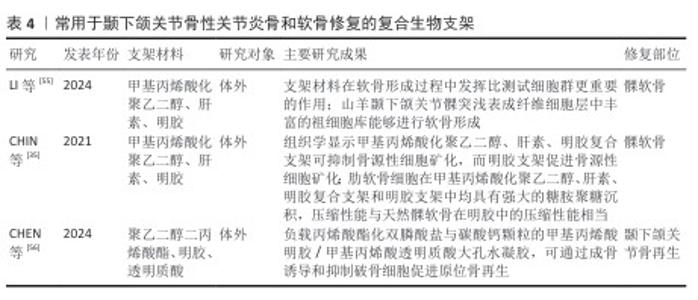
2.2.3 用于颞下颌关节骨关节炎骨和软骨修复的复合支架 复合材料是指是由2种或以上的材料质组合而成。由于天然支架和合成支架各有优缺点,通过化学共轭和聚合将合成支架的机械性能与天然支架的生物特性结合可得到理想的复合材料。复合材料不仅能确保原有材料的基本特性,还可充分发挥它们的生物特性。研究人员合成了由海藻酸盐水凝胶和聚己内酯电纺纤维层组成的复合支架,该支架结构类似于珊瑚和蜘蛛网,具有更高的孔隙率和更好的剪切力学性能,如提高的剪切储存模量和损失模量[53]。 利用透明质酸改性聚乳酸支架来构建同种异体组织工程软骨,在体外与兔髁突软骨细胞共培养后,改性后支架组织骨组织染色和Ⅱ型胶原分泌结果均强于未改性聚乳酸支架[54]。常用于颞下颌关节骨性关节炎骨和软骨修复的复合生物支架,见表4。 "


2.3 水凝胶制造工艺的分类 2.3.1 冷冻干燥 冷冻干燥是制备生物支架的常用方法,在低温、低压的可控条件下操作,可以将天然支架转化为片状结构。冷冻干燥技术可用于制备具有多孔结构的支架,有助于提高支架的生物相容性以及组织工程应用中的细胞生长和营养物质运输[31]。在制备水凝胶过程中,可以通过成分之间的物理或化学交联形成水凝胶,然后通过冷冻干燥得到多孔、干燥的支架。冷冻干燥技术有保留材料固有形态和特性、增强吸湿能力、提高稳定性等优点[57]。 2.3.2 3D打印 生物支架广泛应用于3D打印领域,其中水凝胶主要用于可注射形式的软骨再生,这些可注射制剂能够深入浸润炎症部位,有助于对炎症组织进行更深入和有针对性的治疗,从而有效恢复受损区域的功能[57]。近年来,能够精确设计和制造的3D打印支架技术已成为软骨再生和关节修复的一项非常有前途的技术[9]。在三维环境中培养兔骨髓来源间充质干细胞,并使用拥有强大机械强度和生物相容性的多肽水凝胶进行诱导,可显著增强间充质干细胞向软骨细胞的分化[58]。用于软骨工程的3D支架不仅能满足维持细胞活力的要求,还能实现药物或细胞因子的持续释放,提供足够的结构强度。将3D打印的髁突与含有软骨基质的水凝胶植入山羊髁突中,MicroCT评估显示某些区域的骨生长和组织反应显示出新软骨形成的迹象[41]。 2.3.3 静电纺丝 静电纺丝是一种在强电场中喷射聚合物溶液以生产纳米纤维的制造技术,可制备具有多孔微结构的可生物降解膜和支架。静电纺丝技术被广泛应用于制备富含生长因子的合成或天然支架,但在颞下颌关节中的应用相对较少。电纺丝纳米纤维可以与水凝胶结合形成3D支架,研究人员通过将更坚韧、更多孔的电纺纳米纤维与水凝胶融合创造出3D复合支架[59],这些结构不仅赋予支架了可调节的降解速率,还显著增强了细胞浸润能力,优化了符合组织工程支架标准的机械特性。 "


2.4 水凝胶按搭载的物质种类分类 水凝胶支架优异的结构特性和良好的生物相容性可制备网状和多孔的支架,成为装载细胞因子和生物大分子的理想载体[60]。 2.4.1 搭载种子细胞 与无细胞植入方法相比,支架与细胞或软骨细胞的协同使用显著改善了纤维软骨的再生效果[58],表明细胞/水凝胶再生支架在促进软骨再生方面具有优越性能。将祖细胞接种到改性明胶水凝胶支架中,在成软骨培养基中培养3周可显著促进祖细胞向软骨细胞分化,促进软骨组织的形成[55]。将干细胞加载到具有转化生长因子β缓释功能和超润湿性的明胶水凝胶微球上,动物体内移植后可以有效渗透到颞下颌关节骨缺损的不规则表面,从而加速颞下颌关节缺损区软骨的愈合[61]。将新生儿人真皮成纤维细胞添加到海藻酸盐微球中并进行机械刺激,可改善软骨分化[62]。在明胶基底中培养的下颌髁软骨细胞可观察到糖胺聚糖沉积,但在由甲基丙烯酸化聚乙二醇、肝素和明胶组成的复合水凝胶中培养的下颌髁软骨细胞不能形成糖胺聚糖;而肋软骨细胞在复合水凝胶和明胶中均表现出强大的糖胺聚糖沉积,这表明不同来源细胞在不同支架成分中的表现不一致[35]。采用可注射的自交联巯基透明质酸/ Ⅰ型胶原混合水凝胶结合兔骨髓间充质干细胞和软骨细胞制备新型双层支架,可修复兔髁突骨软骨缺损[18]。透明质酸水凝胶与含有间充质干细胞生物活性成分的条件培养基相结合,植入动物体内后可以显著改善颞下颌关节骨关节炎的关节表面,并且椎间盘厚度、纤维软骨层数、小梁骨量、胶原含量等参数均有显著改善[28]。 2.4.2 搭载生长因子 生长因子是多种细胞产生的低分子质量可溶性蛋白质,具有调节免疫、影响细胞生成、生长以及损伤组织修复等多种功能。近年来,通过局部注射生长因子使得关节组织修复再生的补充治疗方法在关节治疗中受到青睐。近年来,与颞下颌关节支架复合应用的活性因子主要是转化生长因子β家族。将纤维软骨细胞和关节软骨细胞共培养所形成的支架负载生长因子,可形成与天然颞下颌纤维软骨结构和细胞外基质组成类似的支架[63]。AHTIAINEN等[64]在体外用双层聚丙交酯关节盘荷载自体脂肪干细胞和转化生长因子β1,在培养基中培养1周后植入到颞下颌关节盘中,复合支架修复的关节盘未出现明显关节炎症及髁突肥大。用聚d,l-乳酸-羟基乙酸共聚物微球荷载骨形态发生蛋白2和转化生长因子β1,基于梯度渐变的合成方法以模仿骨至软骨的过渡,可修复兔髁突骨和软骨缺损[65]。将结缔组织生长因子、转化生长因子β3封装到聚己内酯支架上中,动物体内移植后可显著促进穿孔关节盘的愈合[66]。临床用于颞下颌关节的生长因子还有富血小板衍生物和浓缩生长因子,但目前暂无与支架复合使用的研究,未来的研究可注重优化生长因子与材料结合使用以延长其在体内环境中的长期效果,提升颞下颌关节骨与软骨的修复效果。 "


2.5 常用于颞下颌关节的支架材料 近年来,水凝胶和脱细胞外基质在颞下颌关节炎修复中展现出广阔的应用前景,可精准模拟颞下颌关节复杂的解剖结构。水凝胶具有高含水率、良好的生物相容性和可调的力学性能,能模拟关节软骨的微环境,促进软骨细胞增殖与分化,适用于颞下颌关节盘缺损的填充与再生。脱细胞外基质材料通过保留天然细胞外基质的拓扑结构和生物活性成分,为细胞迁移和组织再生提供仿生支架,常用于颞下颌关节骨和软骨复合缺损的修复。 2.5.1 水凝胶 水凝胶是一种通过物理或化学交联形成的三维网状结构的交联聚合物,能够通过提供特定性能和模拟不同形态来满足机械需求,并在水中具有优异的膨胀性。水凝胶有助于组织的生成与再生,以满足生物相容性和生物降解等多样化生物需求,同时支持细胞黏附、增殖及分化。用于颞下颌关节修复的水凝胶需要具有一定的物理化学性能,如适当的孔隙率和孔径以利于细胞接种以及细胞和营养物质在支架结构中的自由扩散;还需具有与组织形成相匹配的生物降解速度和能提供组织支撑的机械性能。在组织工程支架材料及药物控制释放方面,水凝胶得到了广泛应用,但水凝胶刚度低、易降解,可能导致结构崩塌或制造受限,因此水凝胶的结构和功能优化还需进一步研究。可以通过pH值或固体含量来调节水凝胶的凝胶化时间和机械性能[9]。聚2-丙烯酰胺基-2-甲基丙磺酸和(3-丙烯酰胺丙基)三甲基氯化铵合成的三重网络水凝胶,可以协同利用网络内静电排斥和疏水相互作用以及网络间静电吸引相互作用来增加模量,获得了模量匹配的软骨替代水凝胶[67]。通过循环冻融交联形成的聚乙烯醇水凝胶和通过3D打印的聚己内酯植入物可加强水凝胶机械性能,12周后可诱导颞下颌关节盘缺损修复[52]。DELIOGULLARI等[68]合成的可注射肝素偶联泊洛沙姆水凝胶具有高弹性和良好的流变特性,有利于软骨细胞增殖。综上所述,水凝胶在颞下颌关节骨和软骨修复中展现出显著优势,能够有效促进颞下颌关节组织再生。 2.5.2 脱细胞基质材料 由脱细胞基质制备的支架在组织工程领域被公认为既经济又高效的一种材料,它们可以保留生物活性成分的能力,并且在最佳条件下可以复制天然细胞外基质的特性,因此这些支架提供了促进细胞生长和分化的关键微环境[69]。天然颞下颌关节关节盘脱细胞支架具有良好的生物活性,并保持源组织优异的力学性能[70]。然而有证据表明,脱细胞过程改变了细胞外基质的结构,使细胞外基质纤维更加致密,从而增加了压缩模量并降低了渗透性,导致细胞分布不均匀[71-72]。由于水凝胶结构松散、降解速度较快,制备脱细胞组织支架可以克服这一问题,但快速降解可能导致支架无法提供长期适当的稳定性。3D打印技术被用于制造由聚己内酯/聚氨酯复合材料制成的支架以及仅由聚氨酯组成的支架,以复制颞下颌关节盘固有的独特生物力学特征,当与脱细胞细胞外基质结合时这些制造的支架提供了有利的物理化学微环境,可以增强细胞的附着、生长、分化和组织修复[46]。脱细胞支架的制备条件会影响支架的力学性能,将猪颞下颌关节椎间盘经连续洗涤和酶处理制成脱细胞细胞外基质,该细胞外基质经胃蛋白酶消化加工成水凝胶,这种水凝胶具有注射性能,在37 ℃下可快速凝胶化[73]。脱细胞支架保留了组织特异性结构、生物学和生物力学特性,在颞下颌关节炎的骨与软骨修复和再生应用中拥有巨大潜力。但在脱细胞基质支架处理过程中部分细胞外基质和机械性能损失,因此,如何更好地优化组织脱细胞处理方法,将脱细胞基质与其他支架材料相结合来实现颞下颌关节炎骨和软骨的修复仍有待进一步研究。 "

| [1] MÉLOU C, PELLEN-MUSSI P, JEANNE S, et al. Osteoarthritis of the Temporomandibular Joint: A Narrative Overview. Medicina. 2022;59(1):8. [2] RENTSCH M, ZUMBRUNN WOJCZYŃSKA A, GALLO LM, et al. Prevalence of Temporomandibular Disorders Based on a Shortened Symptom Questionnaire of the Diagnostic Criteria for Temporomandibular Disorders and Its Screening Reliability for Children and Adolescents Aged 7-14 Years. J Clin Med. 2023;12(12):4109. [3] ÂNGELO D F, WANG Y, MOROUÇO P, et al. A randomized controlled preclinical trial on 3 interposal temporomandibular joint disc implants: TEMPOJIMS-Phase 2. J Tissue Eng Regen Med. 2021;15(10):852-868. [4] DERWICH M, MITUS-KENIG M, PAWLOWSKA E. Orally Administered NSAIDs-General Characteristics and Usage in the Treatment of Temporomandibular Joint Osteoarthritis-A Narrative Review. Pharmaceuticals. 2021;14(3):219. [5] CHĘCIŃSKI M, CHĘCIŃSKA K, TUROSZ N, et al. Current Clinical Research Directions on Temporomandibular Joint Intra-Articular Injections: A Mapping Review. J Clin Med. 2023;12(14):4655. [6] ÂNGELO DF, MOTA B, JOÃO RS, et al. Prevalence of Clinical Signs and Symptoms of Temporomandibular Joint Disorders Registered in the EUROTMJ Database: A Prospective Study in a Portuguese Center. J Clin Med. 2023;12(10):3553. [7] XU X, SUI B, LIU X, et al. A bioinspired and high-strengthed hydrogel for regeneration of perforated temporomandibular joint disc: Construction and pleiotropic immunomodulatory effects. Bioact Mater. 2023;25:701-715. [8] YANG F, LI Y, WANG L, et al. Full-thickness osteochondral defect repair using a biodegradable bilayered scaffold of porous zinc and chondroitin sulfate hydrogel. Bioact Mater. 2024;32:400-414. [9] HASANI-SADRABADI MM, SARRION P, POURAGHAEI S, et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med. 2020;12(534):eaay6853. [10] WANG C, WANG Y, WANG C, et al. Therapeutic application of 3B-PEG injectable hydrogel/Nell-1 composite system to temporomandibular joint osteoarthritis. Biomed Mater. 2022;17(1):015004. [11] SONNENBURG I, SONNENBURG M, FETHKE K. Total replacement of the temporomandibular joint using alloplastic material. 2. Stomatol DDR. 1982;32(3):178-185. [12] LANGER R, VACANTI JP. Tissue Engineering. Science. 1993;260(5110):920-926. [13] PUELACHER WC, WISSER J, VACANTI CA, et al. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J Oral Maxillofac Surg. 1994;52(11):1172-1177. [14] SPRINGER ING, FLEINER B, JEPSEN S, et al. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials. 2001;22(18):2569-2577. [15] SMITH MH, FLANAGAN CL, KEMPPAINEN JM, et al. Computed tomography-based tissue-engineered scaffolds in craniomaxillofacial surgery. Int J Med Robot Comput Assist Surg. 2007;3(3):207-216. [16] LUMPKINS SB, PIERRE N, MCFETRIDGE PS. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008;4(4):808-816. [17] WU Y, GONG Z, LI J, et al. The Pilot Study of Fibrin with Temporomandibular Joint Derived Synovial Stem Cells in Repairing TMJ Disc Perforation. BioMed Res Int. 2014; 2014(1):454021. [18] WANG H, XU Y, WANG P, et al. Cell-mediated injectable blend hydrogel-BCP ceramic scaffold for in situ condylar osteochondral repair. Acta Biomater. 2021;123:364-378. [19] JEONG HJ, HOANG LAP, CHEN N, et al. Engineering soft-hard tissue interfaces in dental and craniofacial system by spatially controlled bioactivities. Bioact Mater. 2025; 45:246-256. [20] CHENG MS, YI X, ZHOU Q. Overexpression of HIF-1alpha in Bone Marrow Mesenchymal Stem Cells Promote the Repair of Mandibular Condylar Osteochondral Defect in a Rabbit Model. J Oral Maxillofac Surg. 2021;79(2):345.e1-345.e15. [21] REED DA, ZHAO Y, HAN M, et al. Mechanical Loading Disrupts Focal Adhesion Kinase Activation in Mandibular Fibrochondrocytes During Murine Temporomandibular Joint Osteoarthritis. J Oral Maxillofac Surg. 2021; 79(10):2058.e1-2058.e15. [22] SHI C, YAO Y, WANG L, et al. Human Salivary Histatin-1-Functionalized Gelatin Methacrylate Hydrogels Promote the Regeneration of Cartilage and Subchondral Bone in Temporomandibular Joints. Basel Switz. 2021;14(5):484. [23] DU Y, CHEN M, JIANG J, et al. Hst1/Gel-MA Scaffold Significantly Promotes the Quality of Osteochondral Regeneration in the Temporomandibular Joint. J Funct Biomater. 2023;14(10):513. [24] HELGELAND E, MOHAMED-AHMED S, SHANBHAG S, et al. 3D printed gelatin-genipin scaffolds for temporomandibular joint cartilage regeneration. Biomed Phys Eng Express. 2021;7(5): 055025. [25] MONTEIRO JL, TAKUSAGAWA T, SAMPAIO GC, et al. Gelatin methacryloyl hydrogel with and without dental pulp stem cells for TMJ regeneration: An in vivo study in rabbits. J Oral Rehabil. 2024;51(2):394-403. [26] HELGELAND E, RASHAD A, CAMPODONI E, et al. Dual-crosslinked 3D printed gelatin scaffolds with potential for temporomandibular joint cartilage regeneration. Biomed Mater. 2021; 16(3):035026. [27] ZHAO H. Fabrication of novel nanofiber composed of gelatin/alginate with zirconium oxide NPs regulate orthodontic progression of cartilage degeneration on Wnt/β-catenin signaling axis in MC3T3-E1 cells. Regen Ther. 2024;25:308-319. [28] ASSI MM, GRAWISH ME, ELSABAA HM, et al. Therapeutic potential of hyaluronic acid hydrogel combined with bone marrow stem cells-conditioned medium on arthritic rats’ TMJs. Sci Rep. 2024;14(1):26828. [29] ZHU Y, CAO L, YUAN M, et al. Microgel Encapsulated Mesoporous Silica Nanoparticles for Releasing Wnt16 to Synergistically Treat Temporomandibular Joint Osteoarthritis. Adv Sci Weinh Baden-Wurtt Ger. 2024;11(41):e2404396. [30] RUSCITTO A, CHEN P, TOSA I, et al. Lgr5-expressing secretory cells form a Wnt inhibitory niche in cartilage critical for chondrocyte identity. Cell Stem Cell. 2023; 30(9):1179-1198.e7. [31] DIEZ-GUARDIA V, TIAN Y, GUO Y, et al. Controlled Release of Human Dental Pulp Stem Cell-Derived Exosomes from Hydrogels Attenuates Temporomandibular Joint Osteoarthritis. Adv Healthc Mater, 2024:e2402923. doi: 10.1002/adhm. 202402923. [32] XU X, SUN J. A mini-invasive injectable hydrogel for temporomandibular joint osteoarthritis: Its pleiotropic effects and multiple pathways in cartilage regeneration. Biomater Adv. 2025;169:214162. [33] TALAAT WM, HAIDER M, KAWAS SA, et al. Chitosan-Based Thermosensitive Hydrogel for Controlled Drug Delivery to the Temporomandibular Joint. J Craniofac Surg. 2016;27(3):735. [34] BOUSNAKI M, BAKOPOULOU A, PAPADOGIANNI D, et al. Fibro/chondrogenic differentiation of dental stem cells into chitosan/alginate scaffolds towards temporomandibular joint disc regeneration. J Mater Sci Mater Med. 2018;29(7):97. [35] CHIN AR, TABOAS JM, ALMARZA AJ. Regenerative Potential of Mandibular Condyle Cartilage and Bone Cells Compared to Costal Cartilage Cells When Seeded in Novel Gelatin Based Hydrogels. Ann Biomed Eng. 2021;49(5):1353-1363. [36] ANANTHANARAYANAN B, KIM Y, KUMAR S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials. 2011;32(31):7913-7923. [37] LI HP, SUN SF, FAN BT, et al. Prevention of adhesions in the temporomandibular joint by the use of chitosan membrane in goats. Br J Oral Maxillofac Surg. 2017;55(1):26-30. [38] HEIRANI-TABASI A, HOSSEINZADEH S, RABBANI S, et al. Cartilage tissue engineering by co-transplantation of chondrocyte extracellular vesicles and mesenchymal stem cells, entrapped in chitosan-hyaluronic acid hydrogel. Biomed Mater. 2021;16(5):055003. [39] CHANG J, MA X, WEI M, et al. Application of alginate three-dimensional culture system for in vitro culture of mandibular condylar chondrocytes from human osteoarthritic temporomandibular joint. Chin J Stomatol. 2002;37(4):246-248. [40] LIU Q, LI Q, XU S, et al. Preparation and Properties of 3D Printed Alginate-Chitosan Polyion Complex Hydrogels for Tissue Engineering. Polymers. 2018;10(6):664. [41] NEDRELOW DS, RASSI A, AJEEB B, et al. Regenerative Engineering of a Biphasic Patient-Fitted Temporomandibular Joint Condylar Prosthesis. Tissue Eng Part C Methods. 2023;29(7):307-320. [42] GAN Z, ZHAO Y, WU Y, et al. Three-dimensional, biomimetic electrospun scaffolds reinforced with carbon nanotubes for temporomandibular joint disc regeneration. Acta Biomater. 2022;147:221-234. [43] MOURA C, TRINDADE D, VIEIRA M, et al. Multi-Material Implants for Temporomandibular Joint Disc Repair: Tailored Additive Manufacturing Production. Front Bioeng Biotechnol. 2020;8:342. [44] YU X, HU Y, ZOU L, et al. A bilayered scaffold with segregated hydrophilicity-hydrophobicity enables reconstruction of goat hierarchical temporomandibular joint condyle cartilage. Acta Biomater. 2021;121: 288-302. [45] ZHAO B, WANG H, LIU C, et al. A preliminary study of the mechanical properties of 3D-printed personalized mesh titanium alloy prostheses and repair of hemi-mandibular defect in dogs. J Biomed Mater Res B Appl Biomater. 2024;112(9):e35466. [46] YI P, LIANG J, HUANG F, et al. Composite System of 3D-Printed Polymer and Acellular Matrix Hydrogel to Repair Temporomandibular Joint Disc. Front Mater. 2021;8:621416. [47] FRANCISCO L, MOURA C, VIANA T, et al. Poly(ɛ-caprolactone) and Polyethylene Glycol Diacrylate-based Scaffolds for TMJ Bioengineered Disc Implants. Procedia Manuf. 2017;12:291-297. [48] HOLLOWAY JL, SPILLER KL, LOWMAN AM, et al. Analysis of the in vitro swelling behavior of poly(vinyl alcohol) hydrogels in osmotic pressure solution for soft tissue replacement. Acta Biomater. 2011; 7(6):2477-2482. [49] KUIPER JP, PUTTLITZ CM, RAWLINSON JE, et al. A mechanical evaluation of polyvinyl alcohol hydrogels for temporomandibular joint disc replacement. Front Phy. 2022;10: 928579. [50] HOU Y, JIN M, LIU Y, et al. Biomimetic construction of a lubricious hydrogel with robust mechanics via polymer chains interpenetration and entanglement for TMJ disc replacement. Chem Eng J. 2023; 460:141731. [51] ZHANG X, WANG J, JIN H, et al. Bioinspired Supramolecular Lubricating Hydrogel Induced by Shear Force. J Am Chem Soc. 2018;140(9):3186-3189. [52] JIANG N, YANG Y, ZHANG L, et al. 3D-Printed Polycaprolactone Reinforced Hydrogel as an Artificial TMJ Disc. J Dent Res. 2021; 100(8):839-846. [53] PANG L, SUN P, DONG X, et al. Shear viscoelasticity of electrospinning PCL nanofibers reinforced alginate hydrogels. Mater Res Express. 2021;8(5):055402. [54] 刘春栋,张志光,苏凯,等.透明质酸改性聚乳酸支架组织工程软骨的构建[J]. 广东牙病防治,2012,20(3):124-129. [55] LI W, TABOAS JM, ALMARZA AJ. Chondrogenic potential of superficial versus cartilage layer cells of the temporomandibular joint condyle in photopolymerizable gelatin-based hydrogels. Proc Inst Mech Eng H. 2024; 238(7):741-754. [56] CHEN J, JING Y, LIU Y, et al. Molecularly Imprinted Macroporous Hydrogel Promotes Bone Regeneration via Osteogenic Induction and Osteoclastic Inhibition. Adv Healthc Mater. 2024;13(23):e2400897. [57] WANG X, LIU F, WANG T, et al. Applications of hydrogels in tissue-engineered repairing of temporomandibular joint diseases. Biomater Sci. 2024;12(10):2579-2598. [58] YANG R, WANG X, LIU S, et al. Bioinspired poly (γ-glutamic acid) hydrogels for enhanced chondrogenesis of bone marrow-derived mesenchymal stem cells. Int J Biol Macromol. 2020;142:332-344. [59] HAN S, NIE K, LI J, et al. 3D Electrospun Nanofiber-Based Scaffolds: From Preparations and Properties to Tissue Regeneration Applications. Stem Cells Int. 2021;2021:1-22. [60] Zhang W, Zhang Y, Zhang Y, et al. Adhesive and tough hydrogels: from structural design to applications. J Mater Chem B. 2021;9(30):5954-5966. [61] YANG Y, HUANG C, ZHENG H, et al. Superwettable and injectable GelMA-MSC microspheres promote cartilage repair in temporomandibular joints. Front Bioeng Biotechnol. 2022;10:1026911. [62] SINGH M, PIERPOINT M, MIKOS AG, et al. Chondrogenic differentiation of neonatal human dermal fibroblasts encapsulated in alginate beads with hydrostatic compression under hypoxic conditions in the presence of bone morphogenetic protein-2. J Biomed Mater Res. 2011;98A(3):412-424. [63] KALPAKCI KN, KIM EJ, ATHANASIOU KA. Assessment of growth factor treatment on fibrochondrocyte and chondrocyte co-cultures for TMJ fibrocartilage engineering. Acta Biomater. 2011;7(4):1710-1718. [64] AHTIAINEN K, MAUNO J, ELLÄ V, et al. Autologous adipose stem cells and polylactide discs in the replacement of the rabbit temporomandibular joint disc. J R Soc Interface. 2013;10(85):20130287. [65] DORMER NH, BUSAIDY K, BERKLAND CJ, et al. Osteochondral Interface Regeneration of Rabbit Mandibular Condyle With Bioactive Signal Gradients. J Oral Maxillofac Surg. 2011;69(6):e50-e57. [66] TARAFDER S, KOCH A, JUN Y, et al. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016; 8(2):025003. [67] DEMOTT CJ, JONES MR, CHESNEY CD, et al. Ultra-High Modulus Hydrogels Mimicking Cartilage of the Human Body. Macromol Biosci. 2022;22(11):e2200283. [68] DELIOGULLARI B, ILHAN‐AYISIGI E, CAKMAK B, et al. Synthesis of an injectable heparin conjugated poloxamer hydrogel with high elastic recoverability for temporomandibular joint disorders. J Appl Polym Sci. 2022;139(31):e52736. [69] QIAO S, PEIJIE T, NAN J. Crosslinking strategies of decellularized extracellular matrix in tissue regeneration. Mater Res A. 2024;112(5):640-671. [70] ISAEVA EV, BEKETOV EE, ARGUCHINSKAYA NV, et al. Decellularized Extracellular Matrix for Tissue Engineering (Review). Sovrem Tekhnologii Med. 2022;14(3):57. [71] KORNMULLER A, BROWN CFC, YU C, et al. Fabrication of Extracellular Matrix-derived Foams and Microcarriers as Tissue-specific Cell Culture and Delivery Platforms. J Vis Exp. 2017;(122):55436. [72] RAMPAL A, DE LA FUENTE IF, VU NK, et al. Decellularization and Enzymatic Digestion Methods to Enhance ECM Protein Detection via MALDI-MS Imaging. Anal Chem. 2025; 97(1):886-893. [73] LIANG J, YI P, WANG X, et al. Acellular matrix hydrogel for repair of the temporomandibular joint disc. J Biomed Mater Res B Appl Biomater. 2020;108(7): 2995-3007. |
| [1] | Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970. |
| [2] | Liu Yang, Liu Donghui , Xu Lei, Zhan Xu, Sun Haobo, Kang Kai. Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2072-2080. |
| [3] | Wang Liang, Zhang Xin, He Wei, Wang Jian. Clinical application and prospects of MXene-based materials for the repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5282-5294. |
| [4] | Zhou Xiaohui, Wang Siyi, Zhou Qiyun, He Zhao, Jia Yujuan, Wang Yuanbin, Ma Jianwu, Chen Gang, Zheng Feng, Chu Genglei. Nanohydroxyapatite-polyether carbonate urethane electrospinning membrane promotes bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5134-5142. |
| [5] | Bai Xiangyu, Huo Feng, Hao Yan, Wang Zecheng, Guo Xiaoyu. Platelet-derived growth factor BB-loaded chitosan/reduced graphene oxide scaffold for repairing alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 329-337. |
| [6] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [7] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [8] | Shi Xiaonan, Wu Xuan, Zhang Daxu, Hu Jingjing, Zheng Yazhe, Liu Yutong, Zhao Shuo, Li Weilong, Ye Shujun, Wang Jingyi, Yan Li. Preparation and characterization of 3D printed microstructured silk fibroin scaffold for liver injury repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3618-3625. |
| [9] | Gong Yukang, Ye Gaoqi, Wang Chenhao, Chen Dejin, Gao Wenshan. Effects and mechanisms of natural polyphenol-based hydrogels in promoting bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3675-3686. |
| [10] | Yang Lei, Liu Xinfang, Luo Sidong, Zhang Hongan, Wang Yeyang, Chen Weijian. Different preparation methods of silk fibroin and its application in the construction of small-diameter tissue-engineered blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3694-3701. |
| [11] | Xiong Jiaying, Shen Jieyi, Lyu Jiahong. Characteristics and strategies of 3D-printed biomimetic bioceramic scaffolds for repairing jaw defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3709-3716. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||