Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (2): 528-536.doi: 10.12307/2025.585
Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers
Jiang Kan1, 2, Alimujiang·Abudourousuli1, 2, Shalayiding·Aierxiding1, 2, Aikebaierjiang·Aisaiti1, 2, Kutiluke·Shoukeer1, 2, Aikeremujiang·Muheremu1, 2
- 1Department of Orthopedics, 2Key Laboratory of Orthopedic Regenerative Medicine, the Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China
-
Received:2024-11-06Accepted:2024-12-31Online:2026-01-18Published:2025-07-03 -
Contact:Aikeremujiang · Muheremu, MD, Chief physician, Associate professor, Master’s supervisor, Department of Orthopedics, and Key Laboratory of Orthopedic Regenerative Medicine, the Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China -
About author:Jiang Kan, Associate professor, Department of Orthopedics, and Key Laboratory of Orthopedic Regenerative Medicine, the Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China Alimujiang·Abudourousuli, Master candidate, Department of Orthopedics, and Key Laboratory of Orthopedic Regenerative Medicine, the Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China
CLC Number:
Cite this article
Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
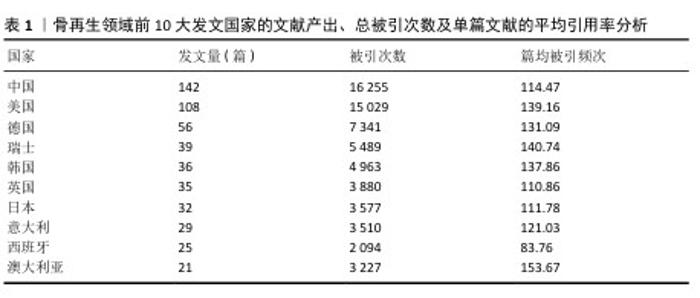
2.2 骨再生领域文献基础量化分析 研究所使用的500篇文献来自于54个国家,683个机构的2 790位作者,发表在141种期刊上,引用了来自2 948种期刊的19 874篇引用文献。在这份涵盖500篇高被引文章的全球研究中,中国以147篇文章的发表量位居榜首,紧随其后的是发表了108篇文章的美国,以及分别发表了56篇和39篇文章的德国和瑞士。就总引用次数而言,中国以16 255次引用和美国以15 029次引用领先,随后是德国的7 341次引用、瑞士的5 498次引用以及韩国的4 963次引用。在引用次数排名前10的国家中,澳大利亚的文章平均引用次数为153.67次,瑞士为140.74次,美国为139.16次,这些数据显示在表1中。此次研究构建了500篇核心引用文献中发文量≥3篇的国家之间合作网络,在这个网络图中,节点大小反映了各国纳入的文章数量,而节点间连线粗细则表示国家间合作的紧密程度。如图2展示,排行榜前10国家之间存在着频繁的合作关系,尤其是中国与美国之间的合作尤为突出。"

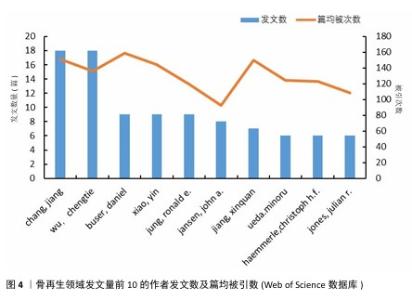
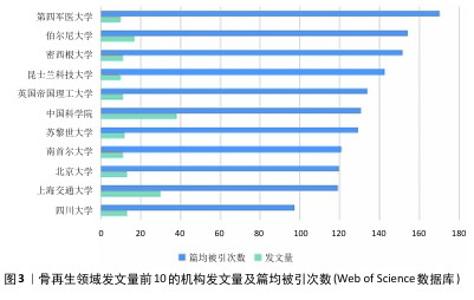
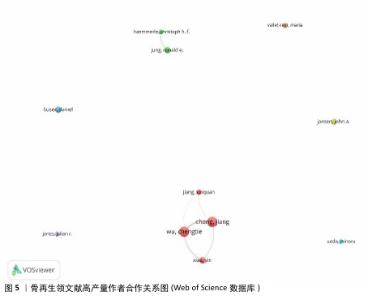
分析文献作者的分布情况,可以识别出特定研究领域的主要学者和关键研究团队。根据Price在1963年的研究,通常在某一特定主题下,一半的论文由一群高产量的作者产出,这个作者群体的规模大约是所有作者总数的平方根。根据Price的公式: 其中,n(x)表示发表x篇论文的作者数量,I=nmax是该领域内最多产作者的论文数(通过VOSviewer统计得知nmax=18),N 是作者总数,m是核心作者的最低发文数。根据Price定律,某一研究领域的核心作者最低发文数m 大约为0.749×nmax,计算得出m=3.17。因此,将发表3篇或以上论文的作者视为该领域的核心作者,共计87名,他们共发表了365篇论文,占总发表量的73%,超过了Price提出的50%门槛。将这些数值代入公式计算,也基本符合Price定律,表明骨科和材料科学领域已经形成了一个相对稳定的合作作者网络。图4展示了发文量前10的作者及其篇均被引次数。从图5可以看出,Chang Jiang、Wu Chengtie、Xiao Yin和Jiang Xinquan等4位作者之间建立了紧密的合作关系,特别是Chang Jiang和Wu Chengtie,他们的发文数量和篇均被引次数都位于前列,具体数据见图4。Chang Jiang和Wu Chengtie是合作密切的中国科学院上海硅酸盐研究所研究员,两位学者在硅酸钙锂体系新型生物活性陶瓷支架及其制备方法和用途方面的研究合作密切,除此之外,在骨再生领域的研究方向有多孔中孔生物玻璃支架、杂化水凝胶、3D打印支架等,两位学者在生物材料和组织工程领域有着显著的研究成果。 "

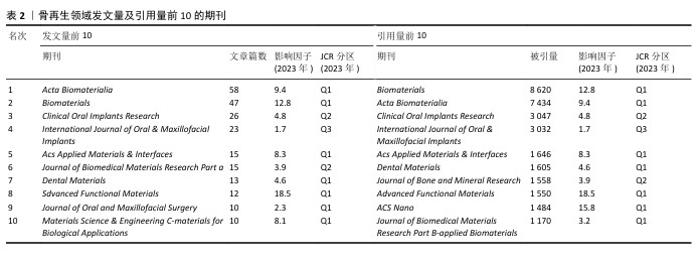
2.4 骨再生领域文献收录期刊分析 此次研究涵盖的500篇文献分布于141种不同的学术期刊中,其中发表数量最多的前10大期刊共收录了229篇论文,占总数的45.8%,具体数据详见表2。在这些期刊中,《Acta Biomaterialia》以58篇论文(占该期刊论文总数的41.1%)位居榜首,随后分别是《Biomaterials》的47篇(33.3%)、《Clinical Oral Implants Research》的26篇(18.4%)、《International Journal of Oral & Maxillofacial Implants》的23篇(16.3%)、《ACS Applied Materials & Interfaces》的15篇(10.6%)。在发文数量排名前10的期刊中,有一半的影响因子超过了5分,而其中70%的期刊被中国科学院期刊分区表归为Q1区。在被引频次前10的期刊中,《Biomaterials》以8 620频次引用量排名第一,随后分别是《Acta Biomaterialia》的7 434频次、《Clinical Oral Implants Research》的3 047频次、《International Journal of Oral & Maxillofacial Implants》的3 032频次、《ACS Applied Materials & Interfaces》的1 646频次。在骨再生和材料科学领域,排名前10的期刊均有超过1 000次的引用频率,并且半数期刊的影响因子超过了5分。"

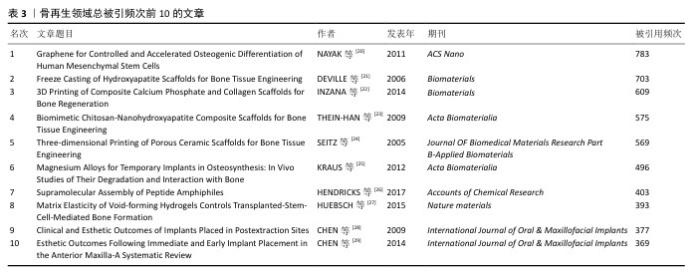
2.5 骨再生领域高引用文章分析 如表3所示,在500篇文献中,引用次数排名前10的文章引用次数均超过了350,而这一顶尖组别的10篇文章发表于2005-2017年间,其中有20%发表在《Biomaterials》,20%发表在《International Journal of Oral & Maxillofacial Implants》,发表在《ACS Nano》上的“Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells”是被引量最多的文章,该文章自2011年发表以来总被引用了783次,其次是2006发表在《Biomaterials》上的文章“Freeze Casting of Hydroxyapatite Scaffolds for Bone Tissue Engineering”和2014同样在《Biomaterials》上发表的文章“3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration”。"

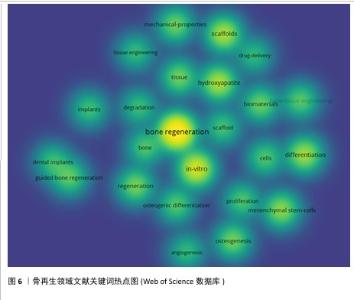
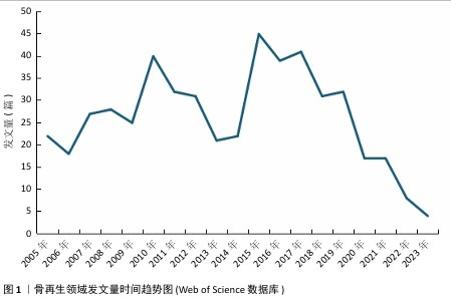
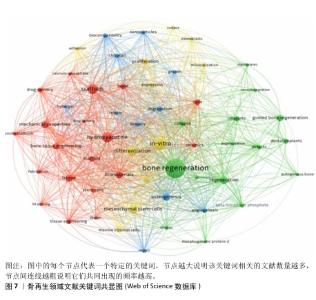
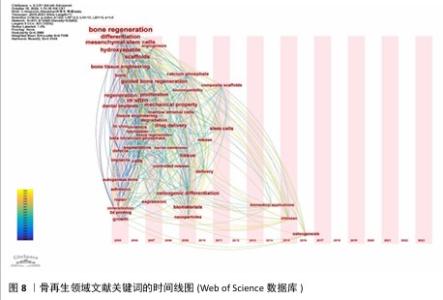
2.6 骨再生领域文献关键词分析与主题趋势 关键词作为文章核心理念的体现,通过观察它们的出现频次,人们能够识别出特定学术领域的关注焦点及其演进方向。在此次分析的500篇文献中,共识别出2 441个关键词,其中有100个关键词的出现次数超过了10次,而有24个关键词的出现频率超过了30次。“骨再生”是出现次数最多的关键词,总计出现了174次,紧随其后的关键词包括“体外”(103次)、“羟基磷灰石”(77次)、“支架”(71次)和“间充质干细胞”(62次),这些频繁出现的关键词揭示了当前骨再生领域的研究重点。利用VOSviewer分析得出出现频次高的关键词热点图,见图6,围绕着最高频的骨再生,除了羟基磷灰石、支架和间充质干细胞外还有生物材料、成骨作用、骨组织工程等。利用VOSviewer做出关键词共显图,见图7,能反映出各关键词之间的共显热度。凭关键词热点图与共显图能得到以下信息:①技术焦点:图中多次提及的3D打印、支架设计、药物递送系统、干细胞疗法等,表明这些是该领域的技术焦点;②材料类型:图中列出了多种生物材料,如钙磷酸盐、水凝胶、壳聚糖、羟基磷灰石等,这些材料在骨组织工程中的运用凸显了材料科学在骨再生研究中的关键角色;③干细胞、骨髓基质细胞、间充质干细胞等细胞类型的频繁出现,凸显了细胞治疗在骨再生中的重要性;④骨形态发生蛋白2等生物活性分子的提及,显示了生长因子和生物活性分子在推动组织再生中的关键作用;⑤生物材料的生物相容性:生物相容性是生物材料设计中的一个重要考虑因素,图中的“biocompatibility”一词强调了这一点。 通过时间序列和引文评价分析关键词,能够揭示研究领域内主题演变的动态趋势。此次研究使用CiteSpace绘制该研究中关键词的时间线图(图8),可以看出,2005-2006年骨再生领域就出现了间充质干细胞、陶瓷、自体骨、羟基磷灰石、3D打印等众多热点话题,到2009年时生物材料、成骨分化是热点话题,到2015年壳聚糖又是一个热点话题,根据图中各关键词之间的连线、连线的粗细和连线颜色浅深能看出新出现的复合材料,壳聚糖、生物材料等热点都与骨再生、间充质干细胞、羟基磷灰石等有密切联系,说明骨再生领域在2005-2006年取得的突破是广泛被同时代以及后来的研究者们认可的,后来的发展趋势也一直是在早期研究者的发现下延续发展并衍生出新领域新材料 。 "

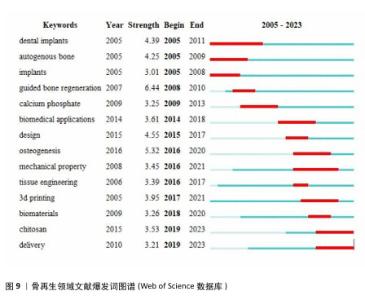
2.7 骨再生领域文献爆发词分析 利用CiteSpace的突发词探测功能可以更清晰地揭示骨再生和材料学领域中新出现的研究方向和研究热点,见图9所示。 爆发词可以被理解为在某个特定时期内,某个词汇或短语突然变得非常流行或被广泛提及的现象。这些数据不仅能够反映出研究领域的热点是如何随着时间推移而变化的,而且还能够揭示近年来研究的发展方向,甚至可能预测未来研究的走向。自2005年以来,自体骨和植入物等相关学科引起了研究者极大的兴趣,以及在后来的几年也保持着研究热度,例如,URBAN等[30]评估颗粒自体骨移植物垂直引导骨再生的结果,在2009年时也被众多研究者关注;2014,2015年,成骨作用、生物医学应用等关键词迎来了爆发期,这在某种意义上是2007年引导性骨再生领域爆发的持续。此外,值得特别关注的一点是,从2018年以来,生物材料、壳聚糖、组织工程等爆发词的出现似乎预示着新型材料的发现与应用是骨再生领域的一关键发展方向以及趋势,其实从2005年起除了自体骨或植入物等一直有与材料学有关的爆发词出现,例如碳酸钙、力学性能、设计、3D打印等均是如此。 "

| [1] EL-RASHIDY AA, ROETHER JA, HARHAUS L, et al. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1-28. [2] SALHOTRA A, SHAH HN, LEVI B, et al. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 2020;21(11): 696-711. [3] WEI J, CHEN X, XU Y, et al. Significance and considerations of establishing standardized critical values for critical size defects in animal models of bone tissue regeneration. Heliyon. 2024;10(13):e33768. [4] ORYAN A, ALIDADI S, MOSHIRI A, et al. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9(1):18. [5] HOLMES D. Non-union bone fracture: a quicker fix. Nature. 2017;550(7677):S193. [6] DIMITRIOU R, JONES E, MCGONAGLE D, et al. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. [7] AHLMANN E, PATZAKIS M, ROIDIS N, et al. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84(5):716-720. [8] ST JOHN TA, VACCARO AR, SAH AP, et al. Physical and monetary costs associated with autogenous bone graft harvesting. Am J Orthop. 2003;32(1):18-23. [9] YOUNGER EM, CHAPMAN MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192-195. [10] FERNANDEZ DE GRADO G, KELLER L, IDOUX-GILLET Y, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng. 2018; 9:2041731418776819. [11] ZHAO Y, CAO G, WANG Z, et al. The recent progress of bone regeneration materials containing EGCG. J Mater Chem B. 2024; 12(39):9835-9844. [12] ELGALI I, OMAR O, DAHLIN C, et al. Guided bone regeneration: materials and biological mechanisms revisitedEuropean. Eur J Oral Sci. 2017;125(5):315-337. [13] EINHORN TA, GERSTENFELD LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45-54. [14] 历龙飞,张朝旭,张馨丹,等.先进材料在骨缺损修复中的应用研究进展[J].中华骨与关节外科杂志,2022,15(1):63-69. [15] MOU J, CUI Y, KURCZ K, et al. Bibliometric and visualized analysis of research on major e-commerce journals using Citespace. JECR. 2019;20(4):219-237. [16] MAYR P, SCHARNHORST A. Scientometrics and information retrieval: weak-links revitalized. Scientometrics. 2014;102:2193-2199. [17] CHEN C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci. 2006;57(3):359-377. [18] VAN ECK NJ, WALTMAN L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523-538. [19] DING X, YANG Z. Knowledge mapping of platform research: a visual analysis using VOSviewer and CiteSpace. Electron Commer Res. 2020;22(4):787-809. [20] NAYAK TR, ANDERSEN H, MAKAM VS, et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano. 2011; 5(6):4670-4678. [21] DEVILLE S, SAIZ E, TOMSIA AP, et al. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials. 2006; 27(32):5480-5489. [22] INZANA JA, OLVERA D, FULLER SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014; 35(13):4026-4034. [23] THEIN-HAN WW, MISRA RD. Biomimetic chitosan-nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009;5(4):1182-1197. [24] SEITZ H, RIEDER W, IRSEN S, et al. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005; 74(2):782-788. [25] KRAUS T, FISCHERAUER SF, HÄNZI AC, et al. Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone. Acta Biomater. 2012;8(3):1230-1238. [26] HENDRICKS MP, SATO K, PALMER LC, et al. Supramolecular Assembly of Peptide Amphiphiles. Acc Chem Res. 2017;50(10): 2440-2448. [27] HUEBSCH N, LIPPENS E, LEE K, et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14(12):1269-1277. [28] CHEN ST, BUSER D. Clinical and esthetic outcomes of implants placed in postextraction sites. Int J Oral Maxillofac Implants. 2009;24 Suppl:186-217. [29] CHEN ST, BUSER D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla-a systematic review. Int J Oral Maxillofac Implants. 2014;29 Suppl:186-215. [30] URBAN IA, JOVANOVIC SA, LOZADA JL. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: a retrospective study of 35 patients 12 to 72 months after loading. Int J Oral Maxillofac Implants. 2009;24(3):502-510. [31] WANG Y, ZHANG H, HU Y, et al. Bone repair biomaterials: a perspective from immunomodulation. Adv Funct Mater. 2022;32(51):2208639. [32] 王子瑞,朱金亮,何志敏,等.人工合成骨修复材料的临床应用及展望[J].生物骨科材料与临床研究,2021,18(4):8-17. [33] KOLK A, HANDSCHEL J, DRESCHER W, et al. Current trends and future perspectives of bone substitute materials - from space holders to innovative biomaterials. J Craniomaxillofac Surg. 2012;40(8):706-718. [34] ŽIVADINOVIĆ M, ANDRIĆ M, MILOŠEVIĆ V, et al. Histomorphometric evaluation of bone regeneration using autogenous bone and beta-tricalcium phosphate in diabetic rabbits. Vojnosanit Pregl. 2016;73(12): 1132-1138. [35] XU S, LIN K, WANG Z, et al. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials. 2008;29(17):2588-2596. [36] LI M, MA H, HAN F, et al. Microbially catalyzed biomaterials for bone regeneration. Adv Mater. 2021;33(49): e2104829. [37] YANG Z, XUE J, SHI Z, et al. Naturally derived flexible bioceramics: Biomass recycling approach and advanced function. Matter. 2024;7(3):1275-1291. [38] GAO C, DENG Y, FENG P, et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci. 2014;15(3):4714-4732. [39] 陈旭卓,周知航,郑吉驷,等.3D生物打印技术在口腔颌面部骨组织缺损修复的研究进展[J].中国口腔颌面外科杂志, 2018,16(3):279-283. [40] PATI F, SONG TH, RIJAL G, et al. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 2015;37:230-241. [41] IVANOVSKI S, BREIK O, CARLUCCIO D, et al. 3D printing for bone regeneration: challenges and opportunities for achieving predictability. Periodontol 2000. 2023;93(1):358-384. [42] 张孝利.GelMA水凝胶在骨组织工程中的研究进展[J].复旦学报(医学版), 2021,48(6):847-851. [43] BEKTAS C, MAO Y. Hydrogel Microparticles for Bone Regeneration. Gels. 2023;10(1):28. [44] HUEBSCH N, LIPPENS E, LEE K, et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14(12): 1269-1277. [45] COSTA-PINTO AR, REIS RL, NEVES NM. Scaffolds based bone tissue engineering: the role of chitosan. Tissue Eng Part B Rev. 2011;17(5):331-347. [46] SARAVANAN S, CHAWLA A, VAIRAMANI M, et al. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int J Biol Macromol. 2017;104(Pt B):1975-1985. [47] THORWARTH M, SCHULTZE-MOSGAU S, KESSLER P, et al. Bone regeneration in osseous defects using a resorbable nanoparticular hydroxyapatite. J Oral Maxillofac Surg. 2005;63(11):1626-1633. [48] BHAT S, UTHAPPA UT, ALTALHI T, et al. Functionalized Porous Hydroxyapatite Scaffolds for Tissue Engineering Applications: A Focused Review. ACS Biomater Sci Eng. 2022;8(10):4039-4076. [49] 莉魏,保金马,邵金龙,等.羟基磷灰石复合材料在骨组织工程中应用的研究进展[J].四川大学学报(医学版),2021, 52(3):357. [50] LI Y, FU Y, ZHANG H, et al. Natural Plant Tissue with Bioinspired Nano Amyloid and Hydroxyapatite as Green Scaffolds for Bone Regeneration. Adv Healthc Mater. 2022; 11(12):e2102807. [51] DHIVYA S, SARAVANAN S, SASTRY TP, et al. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J Nanobiotechnology. 2015;13:40. |
| [1] | Zhang Qiya, Tong Yixiang, Yang Shijiao, Zhang Yumeng, Deng Ling, Wu Wei, Xie Yao, Liao Jian, Mao Ling. In vitro biocompatibility of graded glass infiltrated ultra-translucent zirconia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 443-450. |
| [2] | Zhang Qian, Wang Fuxia, Wang Wen, Zhang Kun. Characteristic analysis of nanogel composite system and its application strategies in visualization of diagnostic imaging and therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 480-488. |
| [3] | Xu Wenhe, Li Xiaobing, Liu Fang. Functionalized biomimetic mineralized collagen modified orthopedic implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 516-527. |
| [4] | Dong Chao, Zhao Mohan, Liu Yunan, Yang Zeli, Chen Leqin, Wang Lanfang. Effects of magnetic nano-drug carriers on exercise-induced muscle injury and inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 345-353. |
| [5] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||