Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (20): 5304-5311.doi: 10.12307/2026.147
Previous Articles Next Articles
3D-printed biodegradable polyester-based scaffolds in bone regeneration therapy
Tang Hao1, Zhong Qian2, Wu Honghan1, Wu Hengpeng1, Wu Xingkai1, Wa Qingde1
- 1Department of Orthopedics, Second Affiliated Hospital of Zunyi Medical University, Zunyi 563006, Guizhou Province, China; 2Chongqing Yongchuan District Maternal and Child Health Hospital, Chongqing 402160, China
-
Accepted:2025-05-24Online:2026-07-18Published:2025-12-02 -
Contact:Wa Qingde, Chief physician, Professor, Department of Orthopedics, Second Affiliated Hospital of Zunyi Medical University, Zunyi 563006, Guizhou Province, China -
About author:Tang Hao, Physician, Department of Orthopedics, Second Affiliated Hospital of Zunyi Medical University, Zunyi 563006, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 82160577 (to WQD); Guizhou Provincial Science and Technology Plan Project, No. ZK[2021]007 (to WQD); Guizhou Province Outstanding Young Science and Technology Talent Training Project, No. [2021]5613 (to WQD)
CLC Number:
Cite this article
Tang Hao, Zhong Qian, Wu Honghan, Wu Hengpeng, Wu Xingkai, Wa Qingde. 3D-printed biodegradable polyester-based scaffolds in bone regeneration therapy[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5304-5311.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
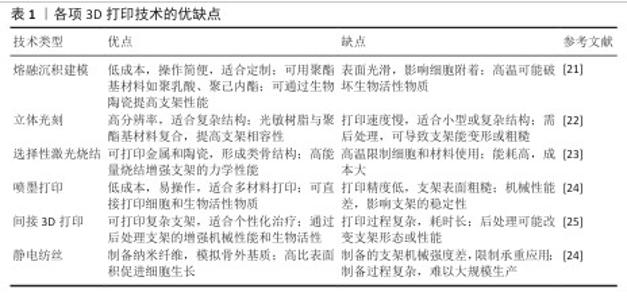
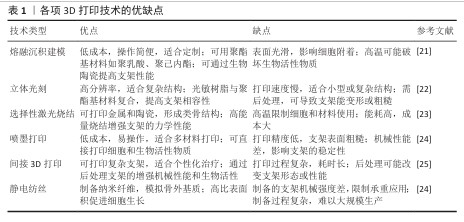
2.1 可降解聚酯基支架特性及3D打印适配性 20世纪30年代,生物可降解聚酯基材料如聚乳酸、聚己内酯、聚乙醇酸、聚乳酸-乙醇酸等,因良好的生物相容性受到广泛关注,并逐渐应用于医疗领域,如制造伤口敷料和外科手术缝线[2]。随着聚酯基材料的开发应用,研究者发现该类材料的降解过程可控,同时具备良好的生物相容性与可调节的机械性能,因此开始尝试将其投入骨修复领域[3]。 当代生物制造技术的迭代革新显著推动了骨修复领域的临床应用进展。基于计算机辅助设计导引的三维建模与离散-叠加式增材制造原理,个性化骨缺损支架可实现亚毫米级精密成型,支架与患者CT三维重建数据的匹配度显著提高[19]。相较于传统成型工艺制备的标准化假体,数字化转型策略可精准控制支架孔隙结构和连通度参数,更契合松质骨再生对微尺度拓扑形貌的需求[20]。现阶段三大主流聚酯基生物支架成型工艺包括熔融沉积成型、选择性激光烧结及立体光刻,它们的典型成型精度分别达到200,100,50 μm量级[7]。尤其以聚己内酯为代表的生物聚合物,因优异的热塑性与光敏特性为复杂多级孔结构的梯度构建提供了良好的物理适配基础。除此之外,喷墨打印、静电纺丝及间接3D打印技术也被应用于聚酯基支架的制备。各项3D打印技术的优缺点,见表1。3D打印技术发展史时间脉络,见图3。 "
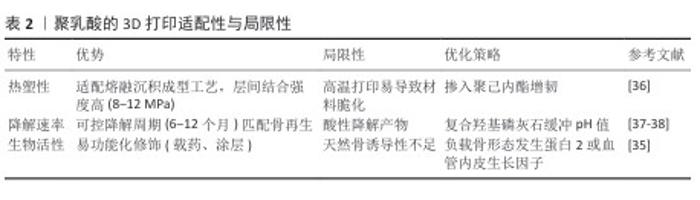
2.1.1 聚乳酸的特性与3D打印适配性 聚乳酸的理化特性与生物相容性:聚乳酸是一种由可再生资源合成的脂肪族聚酯,分子链通过水解或酶解可完全降解为H2O和CO2,被美国食品药品监督管理局及欧盟化学品管理局列为环境友好型医疗材料[26-27]。聚乳酸的降解周期可通过分子质量调控,力学性能与热塑性使它在骨修复领域具备独特优势[27-28]。然而,聚乳酸的疏水表面导致细胞初始黏附率较低[29-30],这一特性虽限制了生物活性,却为它在熔融沉积成型技术中的应用提供了稳定的加工基础。 聚乳酸在熔融沉积成型技术中的工艺适配性:基于聚乳酸的热塑性与可控流变行为,熔融沉积成型技术能够通过180-220 ℃的熔融温度与0.4 mm喷头直径实现高精度打印,层间结合强度达8-12 MPa[28]。通过调控层厚与填充密度可构建孔隙率> 70%、孔径300-500 μm的贯通多孔支架,有效模拟松质骨微结构[31]。例如,WANG等[21]采用熔融沉积成型技术制备聚乳酸/纳米β-磷酸三钙复合支架(质量比7∶3),支架表面粗糙且颗粒分布均匀,表现出松散的多孔结构,植入兔大段骨缺损模型1个月后Micro-CT显示新骨体积占比显著优于对照组。 为克服聚乳酸的疏水性限制,研究者采用碱刻蚀或多巴胺涂层改性,使接触角降低的同时提升细胞黏附率[32-33];此外,掺入20%纳米羟基磷灰石可同步增强支架的压缩模量与骨诱导性[34],而同轴打印负载骨形态发生蛋白2微球则实现28 d缓释,显著促进骨痂形成[35]。未来需进一步优化聚乳酸/聚己内酯共混增韧策略,并探索4D打印温敏响应材料以突破毛细血管级结构的成形限制[7,36]。聚乳酸的3D打印适配性与局限性,见表2。 "

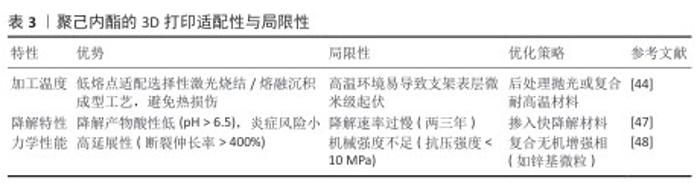
2.1.2 聚己内酯的特性与3D打印适配性 聚己内酯的理化特性与生物相容性:聚己内酯是一种半晶型脂肪族聚酯,低熔点和类橡胶黏弹性使它成为骨修复支架的优选材料[39]。聚己内酯的降解周期较长,通过水解反应生成低酸性代谢产物(pH > 6.5),对局部微环境扰动较小,显著降低炎症反应风险[40-41]。尽管聚己内酯的疏水性限制了细胞初始黏附率[39,42],但它优异的熔融加工性和力学性能使其适配多种3D打印技术[43],尤其是选择性激光烧结和熔融沉积成型。 聚己内酯在3D打印技术中的适配性:①选择性激光烧结工艺:聚己内酯的粉末流动性与低熔点特性适配选择性激光烧结技术,通过调控激光功率和扫描速度可制备孔隙率> 70%、孔径(465±50) μm的贯通多孔支架[44]。例如,DU团队[44]采用选择性激光烧结技术制备由聚己内酯和羟基磷灰石合成的多层次微球软骨支架,该支架支持细胞在体外的黏附、增殖,具有出色的生物相容性且机械性能优异,压缩模量和抗压强度分别达到8.7 MPa和4.6 MPa。此外,选择性激光烧结技术制备表面复合季铵化壳聚糖与氧化石墨烯的聚己内酯/羟基磷灰石支架,展现出协同抗菌与促血管化效应[45]。②熔融沉积成型工艺:聚己内酯的熔融指数适配熔融沉积成型技术的中低温打印(喷头温度80-120 ℃),可构建高延展性支架(断裂伸长率> 400%)[39]。DONG等[46]通过熔融沉积成型技术制备镁掺杂聚己内酯复合支架,该支架的水接触角降至68.4°,其中含质量分数3%镁的复合支架表现出低模量,与人松质骨(50-800 MPa)相匹配,植入大鼠颅骨缺损模型后6周的骨痂矿化速率较纯聚己内酯组显著提高。然而,聚己内酯的疏水性仍制约了它的生物活性,需通过碱刻蚀或表面功能化修饰改善细胞黏附性能[32]。聚己内酯的3D打印适配性与局限性,见表3。"

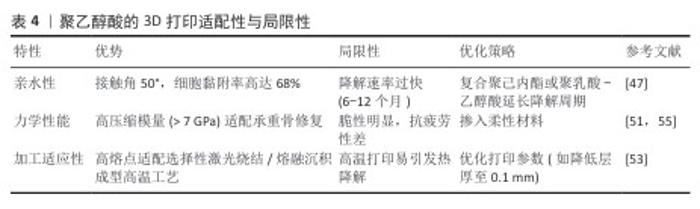
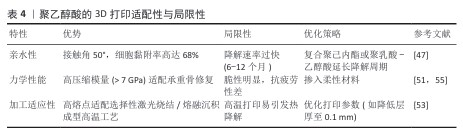
2.1.3 聚乙醇酸的特性与3D打印适配性 聚乙醇酸的理化特性与生物相容性:聚乙醇酸是一种由乙醇酸缩聚而成的全合成聚酯,乙醇酸分子链通过水解反应可完全降解为乙醇酸单体,最终经肾脏代谢排出体外,生物相容性优异[49-50]。聚乙醇酸的结晶度与力学性能使它在骨科内固定器械中应用广泛[51],而亲水性显著优于聚乳酸与聚己内酯[52],可提升间充质干细胞的黏附率[30]。然而,聚乙醇酸的快速降解特性可能导致支架过早崩解,需通过复合策略平衡降解速率与骨再生进程[47]。 聚乙醇酸在3D打印技术中的适配性:①熔融沉积成型工艺:聚乙醇酸的高熔点(225 ℃)与热稳定性使它适配高温熔融沉积成型工艺(喷头温度230-250 ℃)。通过掺入慢降解材料,可构建降解梯度匹配的复合支架。LI等[47]开发的聚乙醇酸/聚己内酯-介孔生物玻璃三元支架(聚乙醇酸∶聚己内酯=50∶50),在缺损模型的生物降解的百分比为 70%-80%,接近新形成骨的百分比( 55%-70%),植入大鼠颌骨缺损模型后12周,Micro-CT显示新骨体积分数较对照组提升。②选择性激光烧结工艺:聚乙醇酸粉末因高结晶度与低吸湿性适配选择性激光烧结技术的激光烧结流程[53]。 SHUAI等[54]通过选择性激光烧结技术制备聚乙醇酸-氢磷灰石支架,支架的抗压强度增加到 95.47 MPa,生物活性较对照组显著提升。聚乙醇酸的3D打印适配性与局限性,见表4。 "
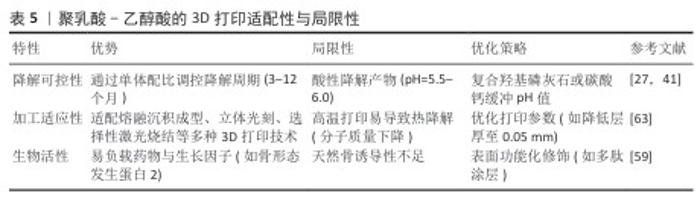
2.1.4 聚乳酸-乙醇酸的特性与3D打印适配性 聚乳酸-乙醇酸的理化特性与生物相容性:聚乳酸-乙醇酸是一种由乳酸与乙醇酸单体共聚而成的可降解聚酯,降解周期可通过单体配比(乳酸∶乙醇酸=75∶25或50∶50)精准调控[56-57]。聚乳酸-乙醇酸兼具聚乳酸的长效稳定性与聚乙醇酸的快速降解性,力学性能和可加工性适配多种3D打印技术[55,58]。聚乳酸-乙醇酸的亲水性显著优于聚乳酸,可提升成骨细胞黏附率至(55±6)%[59],但降解产物酸性可能引发局部炎症反应,需通过复合碱性材料中和[60]。 聚乳酸-乙醇酸在3D打印技术中的适配性:①熔融沉积成型工艺:聚乳酸-乙醇酸的共聚特性(如乳酸∶乙醇酸=75∶25)使它适配中温熔融沉积成型工艺(喷头温度190-210 ℃)。通过掺入硫酸钙或纳米β-磷酸三钙,可显著提升聚乳酸-乙醇酸支架的力学强度与骨诱导性。WANG等[61]开发的3D打印骨形态发生蛋白9 / P-15 肽水凝胶/聚乳酸-乙醇酸复合支架,支架的吸水率约为 9.09%,抗压强度达15.83 MPa,体外细胞实验中成骨相关蛋白含量较对照组更高。②立体光刻工艺:聚乳酸-乙醇酸的光敏改性衍生物(如甲基丙烯酸化聚乳酸-乙醇酸)适配立体光刻技术,可实现超高精度(层厚50 μm)的复杂结构打印。有研究人员用立体光刻技术制备了具有分层结构的纳米聚合物支架(甲基丙烯酰明胶-聚乙二醇二丙烯酸酯-氧化石墨烯支架),实验证明该复合支架的机械性能和生物相容性较好,并且氧化石墨烯诱导人骨髓间充质干细胞软骨分化后,复合支架表面的糖胺聚糖、总蛋白质和胶原蛋白增加了71%,更有利于软骨修复[62]。此外,负载骨形态发生蛋白2的聚乳酸-乙醇酸微球通过立体光刻打印嵌入支架后,支架的抗压强度为16.03 MPa,可维持28 d缓释(累积释放率75%),成骨诱导能力显著提升[55]。聚乳酸-乙醇酸的3D打印适配性与局限性,见表5。 "


2.2 3D打印聚酯支架的优势与瓶颈 2.2.1 核心优势 个性化结构设计:3D打印技术通过计算机辅助设计与医学影像数据(如CT、MRI)结合,可精准构建与患者骨缺损解剖形态匹配的支架。例如,HAN等[64]通过3D打印技术定制聚己内酯支架,将该支架植入到手术切除癌症后复杂上颌缺损患者中,发现植入支架区域的组织密度显著增加。 多级孔隙贯通性:3D打印可通过调控填充密度与孔径,构建仿生松质骨的多级孔结构。LV等[65]采用熔融沉积成型3D 打印技术成功制备了用于松质骨组织工程的多孔磷酸三钙/聚己内酯复合支架,该支架在4%变形下300 s松弛时间后达到24 MPa的平衡应力,平衡模量为428 MPa。 生物活性负载能力:通过同轴打印、微球嵌入或表面涂层技术可精准负载生长因子(如骨形态发生蛋白2、血管内皮生长因子)及药物。LIU等[66]通过同轴3D打印制备负载血管内皮生长因子和骨形态发生蛋白2 的聚 L-乳酸/聚乳酸-羟基乙酸共聚物支架,支架负载的骨形态发生蛋白2能有效促进成骨细胞分化,负载的血管内皮生长因子能促进血管内皮细胞的增殖和分化。SALEHI等[67]通过熔融沉积建模技术制造了含有万古霉素和胰岛素样生长因子1的纳米聚乳酸/聚乙二醇支架,研究表明支架中的万古霉素和胰岛素样生长因子1在PBS中的释放率分别为93.43%和95.86%,可释放28 d,其中万古霉素的释放对金黄色葡萄球菌表现出抗菌活性,形成21.16 mm的抑制区;胰岛素样生长因子1的释放能够抵消万古霉素对细胞行为的不利影响,增强成骨细胞样细胞的黏附和增殖。 材料多样性兼容性:聚酯基材料可与羟基磷灰石、金属微粒(锌、镁)及生物活性玻璃复合,形成多功能支架。例如,LONG等[68]通过3D打印技术制备聚乳酸-乙醇酸/镁多孔支架,该复合支架的压缩模量(89.67±13.99 ) MPa高于聚乳酸-乙醇酸支架的(35.13±8.92) MPa,同时可通过蛋白激酶B和β-catenin 通路促进成骨细胞分化和成骨行为。 2.2.2 当前瓶颈 材料性能限制:聚酯基材料的固有特性限制了其临床转化潜力,聚乳酸与聚己内酯的强疏水性(接触角分别为110°和85°[29,39])导致细胞初始黏附率低下[30],直接影响成骨细胞的功能表达;聚乳酸与聚乳酸-乙醇酸降解产生的酸性副产物(局部pH降至5.5-6.0)可诱发炎症因子(如白细胞介素6)异常升高,抑制骨再生进程[60];聚己内酯的缓慢降解与聚乙醇酸的过快崩解形成鲜明矛盾[40,53],需通过共混或梯度设计实现力学强度与降解速率的动态平衡[47]。 打印技术局限性:现有3D打印技术仍面临多重工艺挑战。例如,熔融沉积成型受限于分辨率[7],难以构建毛细血管级(5-10 μm)仿生结构,而高精度立体光刻技术因光敏树脂的生物相容性不足和设备高成本难以普及[63];高温打印过程(如聚乳酸-乙醇酸喷头温度190-210 ℃)易引发聚合物热降解,导致材料分子质量下降> 20%[63],显著削弱支架的力学稳定性;此外,多材料复合打印时异质界面结合强度不足[34],制约了功能化设计的实现。 临床转化难题:从实验室到临床的跨越仍存在显著障碍。在规模化生产方面,现有3D打印设备效率低下[69],难以满足批量需求;长期安全性评估不足,多数研究仅完成短期动物实验(< 12个月),缺乏降解产物全身代谢数据及远期生物相容性验证[60];标准化体系缺失,个性化支架的形态多样性导致力学、降解等评价标准难以统一,并且各国医疗器械审批流程复杂[70],进一步延缓了临床转化进程。 2.3 3D打印可降解聚酯基支架的未来发展 在材料创新方面,聚酯基支架将从单一功能向多功能复合体系升级,通过开发4D温敏或pH值响应材料实现植入后按需形变与药物控释[71],结合仿生矿化技术与抗菌-成骨双功能设计[31,48],构建兼具力学适配性、生物活性和抗菌效能的多模态动态响应系统。在技术创新层面,高精度多技术联用[7]、纳米级打印及AI驱动的智能化制造系统将突破传统精度与效率矛盾[18,70],配合实时工艺参数反馈(温度、层厚)优化复杂骨缺损的定制化修复方案。临床转化需融合酶响应材料降解-再生同步调控机制[55],依托标准化生物相容性评价和患者分组模板库[36,38],平衡个性化与规模化生产;同时加强长期植入安全性验证[60],补全远期疗效数据以支持监管审批。前沿探索则以细胞/支架一体化打印和类器官仿生构建为方向[62,70],通过模拟骨-血管-神经多元微环境推动功能性全层骨再生,为重度创伤与退行性骨病提供根治性策略。 "

| [1] SCHADE AT, SABAWO M, NYAMULANI N, et al. Functional outcomes and quality of life at 1-year follow-up after an open tibia fracture in Malawi: a multicentre, prospective cohort study. Lancet Glob Health. 2023;11(10):e1609-e1618. [2] DUDA GN, GEISSLER S, CHECA S, et al. The decisive early phase of bone regeneration. Nat Rev Rheumatol. 2023;19(2):78-95. [3] FEI F, YAO H, WANG Y, et al. Graphene Oxide/RhPTH(1-34)/Polylactide Composite Nanofibrous Scaffold for Bone Tissue Engineering. Int J Mol Sci. 2023;24(6):5799. [4] WEI S, MA JX, XU L, et al. Biodegradable materials for bone defect repair. Mil Med Res. 2020;7(1):54. [5] XU C, HONG Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact Mater. 2022;15:250-271. [6] KIRSCH M, HERDER AC, BOUDOT C, et al. Xeno-Free In Vitro Cultivation and Osteogenic Differentiation of hAD-MSCs on Resorbable 3D Printed RESOMER®. Mater Basel Switz. 2020;13(15):3399. [7] BANDYOPADHYAY A, MITRA I, BOSE S. 3D Printing for Bone Regeneration. Curr Osteoporos Rep. 2020;18(5):505-514. [8] WANG H, XU S, FAN D, et al. Multifunctional microcapsules: A theranostic agent for US/MR/PAT multi-modality imaging and synergistic chemo-photothermal osteosarcoma therapy. Bioact Mater. 2021; 7:453-465. [9] MANKAEV BN, KARLOV SS. Metal Complexes in the Synthesis of Biodegradable Polymers: Achievements and Prospects. Materials. 2023;16(20):6682. [10] VLACHOU M, SIAMIDI A, ANAGNOSTOPOULOU D, et al. Modified Release of the Pineal Hormone Melatonin from Matrix Tablets Containing Poly(L-lactic Acid) and Its PLA-co-PEAd and PLA-co-PBAd Copolymers. Polymers. 2022;14(8):1504. [11] KATEBIFAR S, ARUL M, ABDULMALIK S, et al. Novel high-strength polyester composite scaffolds for bone regeneration. Polym Adv Technol. 2023;34(12):3770-3791. [12] LIU J, WU S, MA J, et al. Polycaprolactone/Gelatin/Hydroxyapatite Electrospun Nanomembrane Materials Incorporated with Different Proportions of Attapulgite Synergistically Promote Bone Formation. Int J Nanomedicine. 2022;17:4087-4103. [13] WANG Y, YANG H, LI B, et al. Poly(Butylene Adipate/Terephthalate-Co-Glycolate) Copolyester Synthesis Based on Methyl Glycolate with Improved Barrier Properties: From Synthesis to Structure-Property. Int J Mol Sci. 2022;23(19):11074. [14] STAMNITZ S, KLIMCZAK A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells. 2021;10(8):1925. [15] SAVOJI H, DAVENPORT HUYER L, MOHAMMADI MH, et al. 3D Printing of Vascular Tubes Using Bioelastomer Prepolymers by Freeform Reversible Embedding. ACS Biomater Sci Eng. 2020; 6(3):1333-1343. [16] 朱培丽,白金广,宋子民,等.3D打印股骨髁假体治疗陈旧性Hoffa骨折1例[J].创伤外科杂志,2022,24(12):952-953. [17] QU M, WANG C, ZHOU X, et al. Multi-Dimensional Printing for Bone Tissue Engineering. Adv Healthc Mater. 2021; 10(11):e2001986. [18] HAMA R, ULZIIBAYAR A, REINHARDT JW, et al. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules. 2023;13(2):280. [19] JIN M, CHUNG H, KWON P, et al. Effects of Different Titanium Surfaces Created by 3D Printing Methods, Particle Sizes, and Acid Etching on Protein Adsorption and Cell Adhesion, Proliferation, and Differentiation. Bioengineering (Basel). 2022;9(10):514. [20] WU Y, LIU J, KANG L, et al. An overview of 3D printed metal implants in orthopedic applications: Present and future perspectives. Heliyon. 2023;9(7):e17718. [21] WANG W, LIU P, ZHANG B, et al. Fused Deposition Modeling Printed PLA/Nano β-TCP Composite Bone Tissue Engineering Scaffolds for Promoting Osteogenic Induction Function. Int J Nanomedicine. 2023;18:5815-5830. [22] KHALAF AT, WEI Y, WAN J, et al. Bone tissue engineering through 3D bioprinting of bioceramic scaffolds: a review and update. Life Basel Switz. 2022;12(6):903. [23] XU Y, ZHANG F, ZHAI W, et al. Unraveling of advances in 3D-printed polymer-based bone scaffolds. Polymers. 2022;14(3):566. [24] ZHANG Q, ZHOU J, ZHI P, et al. 3D printing method for bone tissue engineering scaffold. Med Nov Technol Devices. 2023;17: None. doi: 10.1016/j.medntd.2022.100205. [25] FU Z, CUI J, ZHAO B, et al. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J Orthop Transl. 2021;28:118-130. [26] WU Y, GAO X, WU J, et al. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers. 2023; 15(14):3096. [27] SINGHVI MS, ZINJARDE SS, GOKHALE DV. Polylactic acid: synthesis and biomedical applications. J Appl Microbiol. 2019;127(6):1612-1626. [28] CHANG PC, LUO HT, LIN ZJ, et al. Preclinical evaluation of a 3D-printed hydroxyapatite/poly(lactic-co-glycolic acid) scaffold for ridge augmentation. J Formos Med Assoc Taiwan Yi Zhi. 2021;120(4):1100-1107. [29] MATSUI T, ARIMA Y, TAKEMOTO N, et al. Cell patterning on polylactic acid through surface-tethered oligonucleotides. Acta Biomater. 2015;13:32-41. [30] KWON DG, KIM MK, JEON YS, et al. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int J Mol Sci. 2022;23(3):1618. [31] GOLEBIOWSKA AA, NUKAVARAPU SP. Bio-inspired zonal-structured matrices for bone-cartilage interface engineering. Biofabrication. 2022;14(2).doi: 10.1088/1758-5090/ac5413. [32] JANMOHAMMADI M, NOURBAKHSH MS, BAHRAMINASAB M, et al. Effect of Pore Characteristics and Alkali Treatment on the Physicochemical and Biological Properties of a 3D-Printed Polycaprolactone Bone Scaffold. ACS Omega. 2023;8(8):7378-7394. [33] KRUPNIN AE, ZAKIROV AR, SEDUSH NG, et al. Theoretical and Experimental Investigation of 3D-Printed Polylactide Laminate Composites’ Mechanical Properties. Materials. 2023;16(22):7229. [34] WANG C, MENG C, ZHANG Z, et al. 3D printing of polycaprolactone/bioactive glass composite scaffolds for in situ bone repair. Ceram Int. 2022;48(6):7491-7499. [35] YONEDA M, TERAI H, IMAI Y, et al. Repair of an intercalated long bone defect with a synthetic biodegradable bone-inducing implant. Biomaterials. 2005;26(25): 5145-5152. [36] WASYŁECZKO M, SIKORSKA W, CHWOJNOWSKI A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes. 2020;10(11):348. [37] JANG HY, SHIN JY, OH SH, et al. PCL/HA Hybrid Microspheres for Effective Osteogenic Differentiation and Bone Regeneration. ACS Biomater Sci Eng. 2020; 6(9):5172-5180. [38] SAINI P, ARORA M, KUMAR MNVR. Poly(lactic acid) blends in biomedical applications. Adv Drug Deliv Rev. 2016;107:47-59. [39] DWIVEDI R, KUMAR S, PANDEY R, et al. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J Oral Biol Craniofacial Res. 2020;10(1):381-388. [40] HUANG B, YANG M, KOU Y, et al. Absorbable implants in sport medicine and arthroscopic surgery: A narrative review of recent development. Bioact Mater. 2023;31:272-283. [41] SU Y, GAO Q, DENG R, et al. Aptamer engineering exosomes loaded on biomimetic periosteum to promote angiogenesis and bone regeneration by targeting injured nerves via JNK3 MAPK pathway. Mater Today Bio. 2022;16:100434. [42] SAVIĆ L, AUGUSTYNIAK EM, KASTENSSON A, et al. Early development of a polycaprolactone electrospun augment for anterior cruciate ligament reconstruction. Mater Sci Eng C Mater Biol Appl. 2021;129: 112414. [43] PETRETTA M, GAMBARDELLA A, DESANDO G, et al. Multifunctional 3D-Printed Magnetic Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Polymers. 2021;13(21):3825. [44] DU Y, LIU H, YANG Q, et al. Selective Laser Sintering Scaffold with Hierarchical Architecture and Gradient Composition for Osteochondral Repair in Rabbits. Biomaterials. 2017;137:37-48. [45] XUE H, ZHANG Z, LIN Z, et al. Enhanced tissue regeneration through immunomodulation of angiogenesis and osteogenesis with a multifaceted nanohybrid modified bioactive scaffold. Bioact Mater. 2022;18:552-568. [46] DONG Q, ZHANG M, ZHOU X, et al. 3D-printed Mg-incorporated PCL-based scaffolds: A promising approach for bone healing. Mater Sci Eng C Mater Biol Appl. 2021;129:112372. [47] LI J, WANG C, GAO G, et al. MBG/ PGA-PCL composite scaffolds provide highly tunable degradation and osteogenic features. Bioact Mater. 2022;15:53-67. [48] WANG S, GU R, WANG F, et al. 3D-Printed PCL/Zn scaffolds for bone regeneration with a dose-dependent effect on osteogenesis and osteoclastogenesis. Mater Today Bio. 2022;13:100202. [49] XU X, SONG J. Segmental long bone regeneration guided by degradable synthetic polymeric scaffolds. Biomater Transl. 2020;1(1):33-45. [50] DWYER KD, COULOMBE KLK. Cardiac mechanostructure: Using mechanics and anisotropy as inspiration for developing epicardial therapies in treating myocardial infarction. Bioact Mater. 2021;6(7):2198-2220. [51] ZHAO X, HU DA, WU D, et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front Bioeng Biotechnol. 2021;9:603444. [52] AL SUBEH ZY, CHU NQ, KORUNES-MILLER JT, et al. Delivery of eupenifeldin via polymer-coated surgical buttresses prevents local lung cancer recurrence. J Control Release. 2021;331:260-269. [53] KUMAR A, MIR SM, ALDULIJAN I, et al. Load-bearing biodegradable PCL-PGA-beta TCP scaffolds for bone tissue regeneration. J Biomed Mater Res B Appl Biomater. 2021; 109(2):193-200. [54] SHUAI C, SHUAI C, WU P, et al. Characterization and bioactivity evaluation of (polyetheretherketone/polyglycolicacid)-hydroyapatite scaffolds for tissue regeneration. Mater Basel Switz. 2016;9(11):934. [55] ZHAO D, ZHU T, LI J, et al. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact Mater. 2021; 6(2):346-360. [56] ESSA D, KONDIAH PPD, CHOONARA YE, et al. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front Bioeng Biotechnol. 2020;8:48. [57] REDDY MSB, PONNAMMA D, CHOUDHARY R, et al. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers. 2021;13(7):1105. [58] NASEEM R, TZIVELEKIS C, GERMAN MJ, et al. Strategies for Enhancing Polyester-Based Materials for Bone Fixation Applications. Molecules. 2021;26(4):992. [59] JIN S, XIA X, HUANG J, et al. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021;127:56-79. [60] VISAN AI, POPESCU-PELIN G, SOCOL G. Degradation Behavior of Polymers Used as Coating Materials for Drug Delivery-A Basic Review. Polymers. 2021;13(8):1272. [61] WANG X, CHEN W, CHEN Z, et al. Preparation of 3D Printing PLGA Scaffold with BMP-9 and P-15 Peptide Hydrogel and Its Application in the Treatment of Bone Defects in Rabbits. Contrast Media Mol Imaging. 2022:1081957. doi: 10.1155/2022/1081957. [62] SHAH M, ULLAH A, AZHER K, et al. Vat photopolymerization-based 3D printing of polymer nanocomposites: current trends and applications. RSC Adv. 2023;13(2): 1456-1496. [63] MARTÍN-MONTAL J, PERNAS-SÁNCHEZ J, VARAS D. Experimental Characterization Framework for SLA Additive Manufacturing Materials. Polymers. 2021;13(7):1147. [64] HAN HH, SHIM JH, LEE H, et al. Reconstruction of complex maxillary defects using patient-specific 3D-printed biodegradable scaffolds. Plast Reconstr Surg Glob Open. 2018;6(11):e1975. [65] LV X, WANG S, XU Z, et al. Structural mechanical properties of 3D printing biomimetic bone replacement materials. Biomim Basel Switz. 2023;8(2):166. [66] LIU Z, XU Z, WANG X, et al. Preparation and biocompatibility of core-shell microspheres for sequential, sustained release of BMP-2 and VEGF. Biomed Res Int. 2022;2022:4072975. [67] SALEHI S, GHOMI H, HASSANZADEH-TABRIZI SA, et al. Antibacterial and osteogenic properties of chitosan-polyethylene glycol nanofibre-coated 3D printed scaffold with vancomycin and insulin-like growth factor-1 release for bone repair. Int J Biol Macromol. 2025;298:139883. [68] LONG J, ZHANG W, CHEN Y, et al. Multifunctional magnesium incorporated scaffolds by 3D-printing for comprehensive postsurgical management of osteosarcoma. Biomaterials. 2021;275:120950. [69] XIAO X, JIN Y, TAN Y, et al. Investigation of the Effects of Roller Spreading Parameters on Powder Bed Quality in Selective Laser Sintering. Mater Basel Switz. 2022;15(11): 3849. [70] LI J, KIM C, PAN CC, et al. Hybprinting for musculoskeletal tissue engineering. iScience, 2022;25(5):104229. [71] DU R, ZHAO B, LUO K, et al. Shape Memory Polyester Scaffold Promotes Bone Defect Repair through Enhanced Osteogenic Ability and Mechanical Stability. ACS Appl Mater Interfaces. 2023;15(36):42930-42941. |
| [1] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [2] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [3] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [4] | Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970. |
| [5] | Wang Liang, Zhang Xin, He Wei, Wang Jian. Clinical application and prospects of MXene-based materials for the repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5282-5294. |
| [6] | Wang Zitong, Wu Zijian, Yang Aofei, Mao Tian, Fang Nan, Wang Zhigang. Biomaterials regulate microenvironment imbalance for treating spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5321-5330. |
| [7] | Zheng Hao, Zhou Tianqi, Pan Jiazhaо, He Jialin, Zou Zihao, Teng Jianxiang, Xie Mengli, Yang Long, Tian Xiaobin. Sandwich-like nanofiber membrane loaded with salidroside regulates macrophage polarization and promotes angiogenesis in diabetic wounds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5152-5166. |
| [8] | Chen Weifei, Mei Yuandong, Ju Jihui. Repair of infected bone defect with dual-ion time-sequenced release multifunctional hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5188-5200. |
| [9] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [10] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| [11] | Xu Wenhe, Li Xiaobing, Liu Fang. Functionalized biomimetic mineralized collagen modified orthopedic implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 516-527. |
| [12] | Wang Ying, Wang Yawen, Xu Yingjie, Wang Yuanfei, Wu Tong. Preparation of polycaprolactone/low molecular weight fucoidan nanofibers by emulsion electrospinning and assessment of their biocompatibility [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 433-442. |
| [13] | Wang Hao, He Qin, Wang Pingxi, Zhang Jun, Wu Zhilin. Deferoxamine-loaded strontium alginate hydrogel promotes the repair of skull injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3609-3617. |
| [14] | Shi Xiaonan, Wu Xuan, Zhang Daxu, Hu Jingjing, Zheng Yazhe, Liu Yutong, Zhao Shuo, Li Weilong, Ye Shujun, Wang Jingyi, Yan Li. Preparation and characterization of 3D printed microstructured silk fibroin scaffold for liver injury repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3618-3625. |
| [15] | Li Liang, Yang Han, Suo Hairui, Guan Lu, Wang Zhenlin. 3D printed methacrylated gelatin/chitosan scaffolds: evaluation of antibacterial, mechanical properties and cytocompatibility [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3636-3642. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||