Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (20): 5295-5303.doi: 10.12307/2026.742
Previous Articles Next Articles
Repairing bone defects with active ingredients of traditional Chinese medicine combined with hydrogels: successes and challenges
Xu Yawei1, Meng Shilong1, Zhang Xu1, Wang Chengjie1, Yuan Yifeng2, Shi Xiaolin2, Wang Jiao3, Liu Kang2
- 1The Second Clinical Medical School, Zhejiang Chinese Medical University, Hangzhou 310053, Zhejiang Province, China; 2The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310005, Zhejiang Province, China; 3Zhejiang Provincial People’s Hospital (People’s Hospital of Hangzhou Medical College), Hangzhou 310014, Zhejiang Province, China
-
Accepted:2025-07-11Online:2026-07-18Published:2025-12-02 -
Contact:Liu Kang, MD, Associate professor, Chief physician, Doctoral supervisor, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310005, Zhejiang Province, China -
About author:Xu Yawei, Master candidate, The Second Clinical Medical School, Zhejiang Chinese Medical University, Hangzhou 310053, Zhejiang Province, China -
Supported by:National Natural Science Foundation of China, No. 82274272 (to LK); Key Scientific Research Support Project of Zhejiang Chinese Medical University, No. 2025JKZDZC10 (to LK)
CLC Number:
Cite this article
Xu Yawei, Meng Shilong, Zhang Xu, Wang Chengjie, Yuan Yifeng, Shi Xiaolin, Wang Jiao, Liu Kang . Repairing bone defects with active ingredients of traditional Chinese medicine combined with hydrogels: successes and challenges[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5295-5303.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
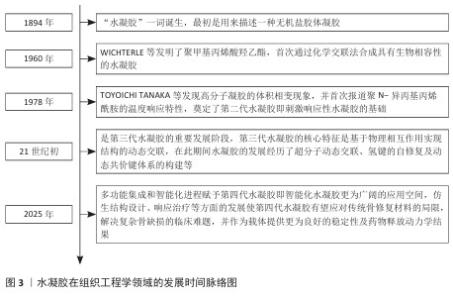
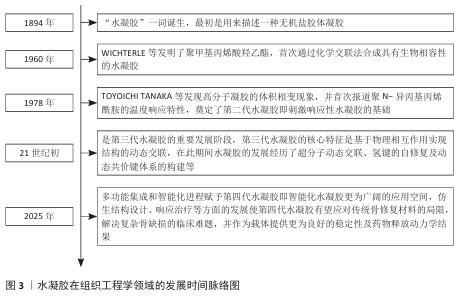
2.1 水凝胶在组织工程学领域的发展 水凝胶在组织工程学领域的发展是一个从基础科学探索到创新技术应用的动态演进过程。“水凝胶”一词诞生于1894年,最初是用来描述一种无机盐胶体凝胶[8]。1960年,WICHTERLE等发明了聚甲基丙烯酸羟乙酯,首次通过化学交联法合成具有生物相容性的水凝胶,虽然是相对简单的合成聚合物,但标志着合成水凝胶在组织工程学领域的兴起和第一代水凝胶的实用化开端[9]。随着功能化与仿生设计的研究深入,TOYOICHI TANAKA等于1978年发现高分子凝胶的体积相变现象,并首次报道聚N-异丙基丙烯酰胺的温度响应特性,奠定了第二代水凝胶即刺激响应性水凝胶的基础,随后包括pH值、离子在内的多种响应类型水凝胶得到应用拓展[8,10]。21世纪初期是第三代水凝胶的重要发展阶段,第三代水凝胶的核心特征是基于物理相互作用实现结构的动态交联,在此期间水凝胶的发展经历了超分子动态交联、氢键的自修复及动态共价键体系的构建等[11]。至今,多功能集成和智能化进程赋予第四代水凝胶(即智能化水凝胶)更为广阔的应用空间,仿生结构设计、响应治疗等方面的发展使第四代水凝胶有望应对传统骨修复材料的局限,解决复杂骨缺损的临床难题,并作为载体提供更为良好的稳定性及药物释放动力学结果[9,12-13]。水凝胶在组织工程学领域的发展时间脉络,见图3。 "
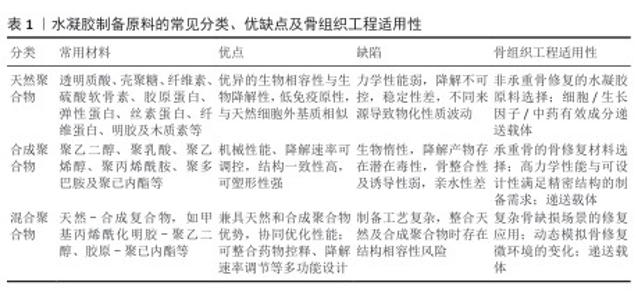
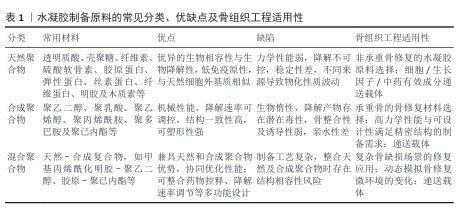
2.2 水凝胶的制备原料 2.2.1 天然聚合物 天然聚合物水凝胶可分类为多糖基水凝胶、蛋白质基水凝胶及芳香族水凝胶。多糖基水凝胶材料主要包括透明质酸、琼脂糖、壳聚糖、海藻酸盐、纤维素、硫酸软骨素及结冷胶。蛋白质基水凝胶的材料则主要包括胶原蛋白、弹性蛋白、丝素蛋白、纤维蛋白及明胶。天然来源的多糖和蛋白质材料在主链中普遍存在大量的羧基(-COOH)、羟基(-OH)、氨基(-NH2)以及醛基(-CHO)官能团,可以直接在水凝胶中形成共价或者非共价的连接[14],从而具备良好的可修饰性。 天然芳香族聚合物是近年来生物组织工程领域兴起的研究热点,其中木质素作为储量最为丰富的天然芳香族聚合物,由对羟苯基丙烷、愈创木基丙烷、紫丁香基丙烷3种单体通过脱氢聚合,来源于不同植物的木质素在3种苯基丙烷单体相对含量上存在着差别[15]。因分子链上含有大量可用作化学改性的活性位点,木质素具备着特殊的结构特征和生物活性[16]。有研究基于木质素磺酸盐和聚乙烯吡咯烷酮制备了一种新型水凝胶,发现木质素赋予生物材料一定的抗菌活性、抗氧化及自愈性能[17]。基于木质素的独特性质,未来可通过官能团的化学修饰及设计获得生物医学应用所需的具备靶向性及均一性的功能化水凝胶。 这些天然高分子材料因良好的生物相容性、高黏附性、生物可降解性、低免疫原性以及最终代谢产物可在体外排泻等诸多优点,成为生物组织工程及递送系统中的理想选择[18]。然而与合成高分子材料相比,纯天然高分子材料在极端条件下缺乏足够的稳定性,构建的水凝胶支架在降解速率调控、可塑性及机械性能方面存在不足。因此,将天然聚合物及合成聚合物通过各种交联方式构建的混合聚合物水凝胶,可以弥补现有的局限并兼具两者的优势性能,是现在的研究方向之一。 2.2.2 合成聚合物 制备合成聚合物水凝胶的材料包括聚乙二醇、聚乙烯醇、聚丙烯酰胺、聚多巴胺及聚己内酯等,通过特定聚合反应所形成的聚合物链决定了水凝胶的特性。其中,水凝胶的机械性能及降解速率等关键因素与合成聚合物自身的化学结构和交联网络的设计密切相关,所以与天然聚合物水凝胶相比,合成聚合物水凝胶能够一定程度地规避天然高分子材料的局限,具有更为广泛的物理化学性质、应用范围及更高的商业可用性[19]。但并非所有聚合物均适用于水凝胶的制备,例如某些聚合物亲水性能的缺失将会严重影响到水凝胶的吸水能力[18]。 2.2.3 混合聚合物 通过整合合成聚合物的物理化学特性和天然高分子材料的固有生物活性,基于适当的交联策略构建的复合水凝胶体系,在组织工程支架及药物递送系统展现出独到的多方面协同优势。LIU等[20]在由透明质酸构建的水凝胶基础上,通过光交联技术融合精氨酸聚合物(酯酰胺)构建了一种新型的混合聚合物水凝胶,发现该水凝胶的内部结构形态、溶胀率、力学及生物降解性能可通过精氨酸聚合物的含量调控,并兼具着天然高分子材料的生物相容性;体外实验证实,随着精氨酸组分的加入,该水凝胶具有良好的抗氧化、抗炎及促血管化功能。综上可见,混合聚合物水凝胶通过“天然-合成协同效应”突破了单一材料在力学、生物活性等方面的瓶颈,拓宽了材料科学与再生医学间的交叉运用领域。文章对水凝胶制备原料的常见分类、优缺点及骨组织工程适用性进行总结,见表1。 "

2.3 中药有效成分对水凝胶性能的改善 2.3.1 生物相容性 生物相容性是指材料与生物体相互作用时正常发挥功能而不引发有害反应的能力。水凝胶的生物相容性是它在生物组织工程领域应用的核心前提[21]。中药有效成分可通过表面修饰、调节细胞外基质的微环境结构等提升水凝胶的生物相容性能。LI等[22]通过实验表明,白藜芦醇的加入可降低热敏水凝胶的免疫原性,同时白藜芦醇可有效清除骨缺损部位的活性氧并降低炎症水平,以此优化热敏水凝胶的生物相容性。另有研究在水凝胶中结合了黄芪多糖成分,发现黄芪多糖在调节免疫反应的同时促进了微环境中成骨细胞的黏附与增殖,为组织修复与再生提供了有益环境[21]。在传统提升材料生物相容性方面的策略中,表面亲水化处理、功能化官能团引入等化学修饰方法以及生物活性因子整合应用广泛。中药有效成分能够与生物材料相互作用促进骨组织再生,同时为改善生物材料的相容性提供了一种创新方法。 2.3.2 机械性能 水凝胶的机械性能是指它在受力作用下的一种响应特性,反映的是材料抵抗变形、维持结构完整并适应环境变化的能力。在骨组织工程研究中,水凝胶的机械性能不仅是修复骨缺损的物理基础,直接影响治疗效果,在微观层面更是调控细胞行为并引导骨再生的关键要素之一。由于特殊的化学特性及结构,中药有效成分在优化水凝胶力学强度、调节孔隙率及韧性等方面显示一定的潜力。DING等[23]研究发现,负载紫杉醇脂质体显著增强了水凝胶的交联密度及物理强度,确保了水凝胶系统在复杂环境下的结构完整与作用稳定。CHATTERJEE等[24]将牡丹皮中的有效成分没食子酸纳入水凝胶制剂中,发现没食子酸改善了水凝胶的机械性能及pH值稳定性。WEI等[25]使用芦荟构建了复合型水凝胶,发现芦荟的加入使水凝胶微观结构更为致密、机械性能及溶胀率得到提升。另有研究利用共价交联法制备了大黄、淫羊藿多糖明胶水凝胶,根据对两种水凝胶性能特征的分析可知,改变两种中药有效成分中的醛基浓度可以获得组织工程学所需的内部结构及强度[26]。这些研究丰富了中药有效成分对于水凝胶力学性能改善的理论及实践基础,为后续两者结合应用提供了广阔的探索方向。 2.3.3 降解性能 水凝胶的降解性能同样影响着骨缺损修复效果。在骨再生领域,水凝胶的降解性能应该动态匹配骨修复进程,降解过程中需要维持必要的机械支撑与生物活性,降解产物需无毒且可被机体排出。ZHANG等[27]利用芦荟多糖改善水凝胶的降解性能,通过减少氧化应激反应并提高交联强度避免水凝胶结构的过早坍塌。降解性能优化是水凝胶在骨再生临床转化的必要挑战,但相关研究处于初步阶段,未来有待进一步深入。"

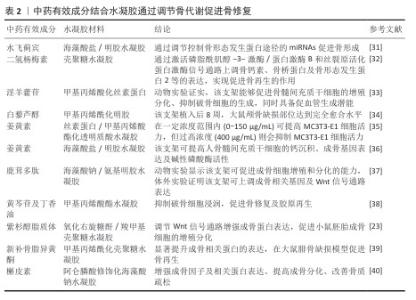
2.4 中药有效成分与水凝胶结合促进骨修复的相关作用 中药来源天然,生物相容性高,具备多靶点调控特性,能够通过多种机制协同作用有效改善骨缺损修复效果。近年来随着生物样本分析技术的发展,中药治疗骨科疾病的成分日渐清晰,推动了中药有效成分的提取与临床应用[28]。因此,中药有效成分在骨组织工程中的应用日益受到关注,中药独特的优势对于骨修复领域的发展具有重要意义。既往研究从活性成分角度对中药进行分类,以此总结中药有效成分搭载支架材料的应用[29],该文拟从中药有效成分的药理作用层面分析,结合相关研究最新进展,从调节骨代谢、促进软骨形成、抑制炎症及氧化应激、血管化与血管生成4个维度阐明中药有效成分结合水凝胶在骨组织工程中的优势。 2.4.1 调节骨代谢 骨代谢过程主要由成骨细胞主导的骨形成和破骨细胞主导的骨吸收调控,骨缺损时损伤部位的微环境会触发一系列复杂的骨代谢动态变化,而中药有效成分主要通过促进成骨细胞的分化与增殖、抑制破骨细胞的形成进行调节[30]。水飞蓟宾是一种植物源性黄酮类化合物,它的生物利用度因疏水性而受限制。LEENA等[31]将负载水飞蓟宾的壳聚糖纳米颗粒纳入基于海藻酸盐-明胶水凝胶支架中,发现从支架中持续释放的水飞蓟宾通过调节控制骨形态发生蛋白途径的miRNAs促进成骨分化。二氢杨梅素同样是从中药提取的一种黄酮类物质,YANG团队[32]在壳聚糖水凝胶中负载二氢杨梅素成分,发现该水凝胶通过激活磷脂酰肌醇-3-激酶/蛋白激酶B和丝裂原活化蛋白激酶信号通路上调骨钙素、骨桥蛋白及骨形态发生蛋白2等的表达,实现促进骨再生作用。LIU等[33]基于三臂主客体超分子、甲基丙烯酸化丝素蛋白以共价交联法合成了一种高强度的水凝胶体系,并将淫羊藿苷负载于其中,生物活性评估及体内实验发现该支架具有促进骨髓间充质干细胞增殖和分化的能力,抗酒石酸酸性磷酸酶染色显示该支架对破骨细胞具有抑制效应,α-平滑肌肌动蛋白表达检测证实该支架具备促血管生成的潜力。WEI等[34]应用甲基丙烯酸明胶水凝胶负载包裹白藜芦醇的固体脂质纳米粒,发现植入该水凝胶支架的大鼠颅骨缺损部位在术后8周达到完全愈合,表现为成骨标志物(碱性磷酸酶、Runt相关转录因子2、骨钙素及骨桥蛋白)显著表达和明显的钙沉积,证实该支架可促进骨髓间充质干细胞的成骨分化。YU等[35]使用丝素蛋白/甲基丙烯酸酯化透明质酸水凝胶负载包裹姜黄素的壳聚糖纳米粒子,成骨细胞体外增殖实验显示,姜黄素在一定浓度范围内(0-150 μg/mL)可提高小鼠胚胎成骨细胞前体细胞(MC3T3-E1)的细胞活力,但过高浓度(400 μg/mL)则会抑制细胞活力,提示中药有效活性成分与生物材料的结合需选择适宜的浓度,以实现骨代谢水平的有效调控。AMIRYAGHOUBI等[36]将包含姜黄素的壳聚糖微球负载于种海藻酸盐/明胶水凝胶支架,与对照组相比,该支架展现出优异的促成骨性,能协同提升人骨髓间充质干细胞的钙结节沉积、碱性磷酸酶活性及成骨基因表达。表2为中药有效成分结合水凝胶通过调节骨代谢促进骨修复的研究总结。综上所述,中药有效成分与水凝胶的复合体系从多角度干预成骨-破骨偶联机制,发挥调节骨代谢作用,但现有研究仅对相关成骨、破骨标志物进行检测,具体信号通路机制仍亟待进一步完善。"
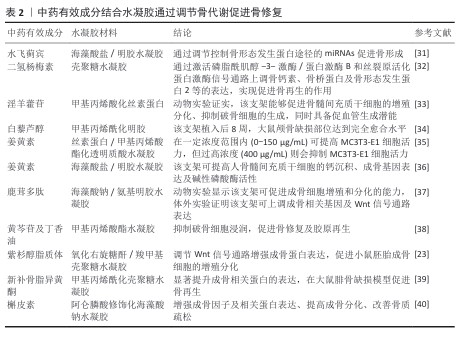
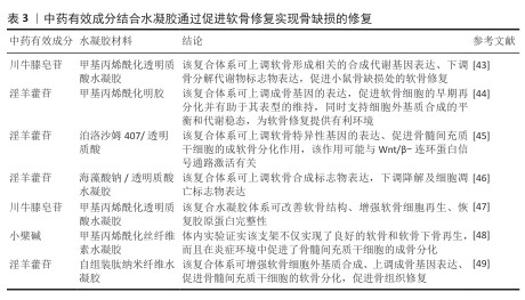
2.4.2 促进软骨形成 软骨内骨形成同样是骨修复的重要途径[41-42]。AN等[43]的动物实验发现,负载川牛膝皂苷A的甲基丙烯酰化透明质酸水凝胶微球提升了与软骨形成相关的合成代谢基因(聚集蛋白聚糖、Ⅱ型胶原α1链和SRY-盒转录因子9)表达,并下调了骨分解代谢物标志物基质金属蛋白酶13的表达,促进骨缺损处的软骨修复。LIAO等[44]将甲基丙烯酸明胶与掺杂镁离子的生物活性玻璃、淫羊藿苷相结合制备复合支架,体外实验证实该支架可显著上调SRY-盒转录因子9、聚集蛋白聚糖、Ⅱ型胶原α1链等成软骨基因的表达,促进软骨细胞的早期再分化并有助于其表型维持,同时支持细胞外基质合成的平衡和代谢稳态,为软骨修复提供了有利环境。ZHU等[45]制备了含有淫羊藿苷的透明质酸/泊洛沙姆407水凝胶,该体系可上调软骨特异性基因的表达、诱导骨髓间充质干细胞的成软骨分化,该作用可能与Wnt/β-连环蛋白信号通路激活有关。LI等[46]在海藻酸钠/透明质酸复合水凝胶中载入淫羊藿苷,该体系可上调软骨合成标志物聚集蛋白聚糖、Ⅱ型胶原α1链表达,下调降解标志物聚集蛋白聚糖酶2、基质金属蛋白酶13、Ⅰ型胶原蛋白α1链及细胞凋亡标志物半胱天冬酶3表达。表3是对中药有效成分结合水凝胶通过促进软骨修复实现骨缺损修复研究的总结。综上所述,软骨内骨形成通过微环境重塑成为骨缺损修复的关键生物学机制之一,中药有效成分与骨组织工程技术的深度融合也将推动个性化骨再生方案的临床转化。 "


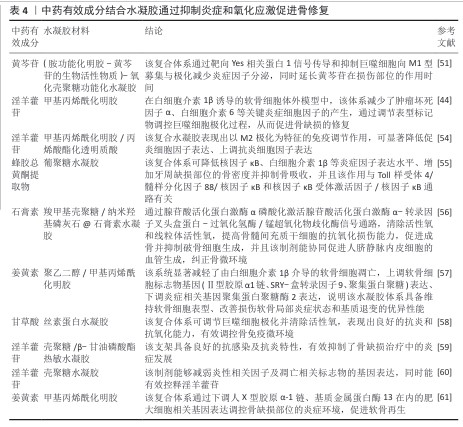
2.4.3 抑制炎症与氧化应激 骨缺损的愈合阶段需要保持微环境中炎症及氧化应激反应的动态平衡,过度或持续的反应将抑制成骨细胞功能,阻碍骨修复进程,进一步加重组织损伤[50],所以,骨缺损治疗中抑制炎症及氧化应激反应起着关键作用。YANG等[51]对负载黄芩苷的氧化硫酸软骨素/明胶水凝胶体系的治疗机制进行探索,发现该制剂通过靶向Yes相关蛋白1信号传导和抑制巨噬细胞向M1型募集与极化减少炎症因子分泌,同时延长了黄芩苷在损伤部位的作用时间。核因子κB是一类与炎症相关的核转录因子,既往研究已表明,淫羊藿苷具有抑制核因子κB信号通路表达的能力[52-53]。LIAO等[44]进一步探讨了淫羊藿苷的抗炎机制,在白细胞介素1β诱导的软骨细胞体外模型中,负载淫羊藿苷的水凝胶体系减少了肿瘤坏死因子α和白细胞介素6等关键炎症细胞因子的产生,通过减少M1表型标志物和增强M2表型标志物来有效调节巨噬细胞极化,从而促进骨缺损的修复。也有研究发现,淫羊藿苷的加入使得水凝胶表现出以M2极化为特征的免疫调节作用,显著降低促炎细胞因子(白细胞介素1β、诱导型一氧化氮合酶)表达、上调抗炎细胞因子(白细胞介素4、白细胞介素1受体拮抗剂)的表达[54]。TANG等[55]在葡聚糖水凝胶中加入蜂胶的黄酮提取物,发现该复合体系可降低核因子κB、白细胞介素1β等炎症因子表达水平、增加牙周缺损部位的骨密度并抑制骨吸收,并且该作用与Toll 样受体4/髓样分化因子88/核因子κB和核因子κB受体激活因子/核因子κB通路有关。ZHENG等[56]开发了一种基于石膏素的可注射复合水凝胶,体外实验证实该制剂通过腺苷酸活化蛋白激酶α磷酸化激活腺苷酸活化蛋白激酶α-转录因子叉头盒蛋白-过氧化氢酶/锰超氧化物歧化酶信号通路,清除活性氧和线粒体活性氧,提高骨髓间充质干细胞的抗氧化损伤能力,促进成骨并抑制破骨细胞生成,并且该制剂能协同促进人脐静脉内皮细胞的血管生成,纠正骨微环境。SUN等[57]基于聚乙二醇-甲基丙烯酰化明胶构建了一种姜黄素递送系统,发现该系统显著减轻了由白细胞介素1β介导的软骨细胞凋亡,上调软骨细胞标志物基因(Ⅱ型胶原α1链、SRY-盒转录因子9、聚集蛋白聚糖)表达、下调炎症相关基因聚集蛋白聚糖酶2表达,说明该水凝胶体系具备维持软骨细胞表型、改善损伤软骨局部炎症状态和基质退变的优异性能。表4总结了中药有效成分结合水凝胶在抑制炎症和氧化应激促进骨修复方面的应用,可见中药有效成分联合水凝胶在为骨缺损再生创造了免疫耐受、氧化还原稳态及力学适应的支持体系。"
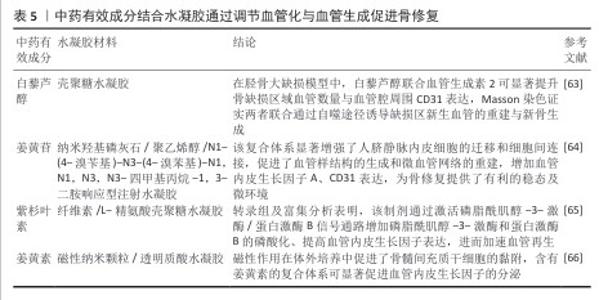
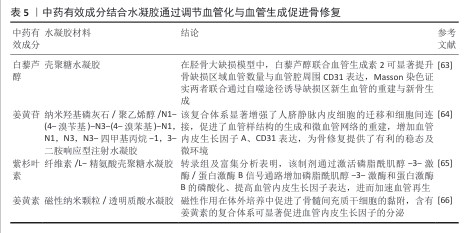
2.4.4 血管化与血管生成 骨骼作为高度血管化的组织,依赖血管以调节营养状态。在骨微环境中,血管生成可以刺激骨细胞的活性,促进骨生成,对于骨修复与重建起着不可或缺的作用[62]。FAN等[63]制备了含有白藜芦醇、血管生成素2及骨髓间充质干细胞的壳聚糖水凝胶支架,将其应用于胫骨大缺损模型中,发现白藜芦醇联合血管生成素2可显著提升骨缺损区域的血管数量、血管腔周围CD31表达,Masson染色证实二者联合通过自噬途径诱导缺损区新生血管的重建与新骨生成。有研究制备了负载姜黄苷的纳米羟基磷灰石/聚乙烯醇/N1-(4-溴苄基)-N3-(4-溴苯基)-N1,N1,N3,N3-四甲基丙烷-1,3-二胺响应型注射水凝胶,发现该水凝胶显著增强了人脐静脉内皮细胞的迁移和细胞间连接,促进了血管样结构的生成和微血管网络的重建,同时随着姜黄苷的持续释放,血管内皮生长因子A、CD31表达明显提高,为骨修复提供了有利的稳态及微环境[64]。YANG等[65]制备了负载紫杉叶素的纤维素/L-精氨酸壳聚糖水凝胶,转录组及富集分析表明,该制剂通过激活磷脂酰肌醇-3-激酶/蛋白激酶B信号通路增加磷脂酰肌醇-3-激酶和蛋白激酶B的磷酸化,促进血管内皮生长因子的表达,进而加速血管再生。DAYA等[66]以姜黄素为血管生成剂,通过一锅共沉淀法将其负载于磁性纳米颗粒中,再与透明质酸的结合构建了一种血管生成磁性水凝胶,磁性作用在体外培养中促进了骨髓间充质干细胞的黏附,该复合水凝胶可显著促进血管内皮生长因子的分泌。表5是对中药有效成分结合水凝胶通过调节血管化与血管生成促进骨修复研究的总结,可见中药有效成分与水凝胶通过自噬、磷脂酰肌醇-3-激酶蛋白激酶B等信号通路途径上调血管生成相关蛋白及活性因子的表达,促进相关细胞的迁移,基于成血管-成骨偶联机制实现骨缺损的修复作用。 "
| [1] HO-SHUI-LING A, BOLANDER J, RUSTOM LE, et al. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180: 143-162. [2] ANNAMALAI RT, HONG X, SCHOTT NG, et al. Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects. Biomaterials. 2019;208:32-44. [3] TANG D, TARE RS, YANG LY, et al. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363-382. [4] 赵恩哲,吴斗,刘强.骨缺损的治疗现状及研究进展[J].中华实验外科杂志,2022, 39(11):2053-2057. [5] CHANG S, WANG S, LIU Z, et al. Advances of Stimulus-Responsive Hydrogels for Bone Defects Repair in Tissue Engineering. Gels. 2022;8(6):389. [6] 熊伟,袁灵梅,钱国文,等.“补肾壮骨”中药应用于骨组织工程支架修复节段性骨缺损 [J]. 中国组织工程研究,2023, 27(21):3438-3444. [7] TANG H, HOSEIN A, MATTIOLI-BELMONTE M. Traditional Chinese Medicine and orthopedic biomaterials: Host of opportunities from herbal extracts. Mater Sci Eng C Mater Biol Appl. 2021;120:111760. [8] NALLUSAMY J, DAS RK. Hydrogels and Their Role in Bone Tissue Engineering: An Overview. J Pharm Bioallied Sci. 2021; 13(Suppl 2):S908-S912. [9] LIU X, SUN S, WANG N, et al. Therapeutic application of hydrogels for bone-related diseases. Front Bioeng Biotechnol. 2022; 10:998988. [10] TOKITA M. Phase Transition of Gels-A Review of Toyoich Tanaka’s Research. Gels. 2022; 8(9):550. [11] STRANDMAN S, ZHU XX. Self-Healing Supramolecular Hydrogels Based on Reversible Physical Interactions. Gels. 2016; 2(2):16. [12] PUSHPARAJ K, BALASUBRAMANIAN B, PAPPUSWAMY M, et al. Out of Box Thinking to Tangible Science: A Benchmark History of 3D Bio-Printing in Regenerative Medicine and Tissues Engineering. Life (Basel). 2023; 13(4):954. [13] DU J, ZHOU T, PENG W. Functional polysaccharide-based hydrogel in bone regeneration: From fundamentals to advanced applications. Carbohydr Polym. 2025;352:123138. [14] PATIL PS, FATHOLLAHIPOUR S, INMANN A, et al. Fluorinated Methacrylamide Chitosan Hydrogel Dressings Improve Regenerated Wound Tissue Quality in Diabetic Wound Healing. Adv Wound Care (New Rochelle). 2019;8(8):374-385. [15] WANG Y, TANG S, JIANG L, et al. A review of lignin application in hydrogel dressing. Int J Biol Macromol. 2024;281(Pt 3):135786. [16] SHAN Z, JIANG B, WANG P, et al. Sustainable lignin-based composite hydrogels for controlled drug release and self-healing in antimicrobial wound dressing. Int J Biol Macromol. 2025;285:138327. [17] CAO JF, ZHAO YN, JIN SC, et al. Flexible Lignin-based hydrogels with Self-healing and adhesive ability driven by noncovalent interactions. Chem Eng J. 2022;429:132252. [18] LIU Z, MA X, LIU J, et al. Advances in the application of natural/synthetic hybrid hydrogels in tissue engineering and delivery systems: A comprehensive review. Int J Pharm. 2025;672:125323. [19] 李艾康,周梓萌,吴凉彬,等.水凝胶支架用于软骨修复的研究进展[J].中华骨与关节外科杂志,2025,18(2):177-183. [20] LIUT , LIU GT, ZHANG JH, et al. l-Arginine based polyester amide/hyaluronic acid hybrid hydrogel with dual anti-inflammation and antioxidant functions for accelerated wound healing. Chin Chem Lett. 2022;33(4):1880-1884. [21] WEI X, XIE H, LIU C, et al. Nature Herbal Medicine- Tissue Engineering Strategies for Regulate Cell Homeostasis in Bone Regeneration. Adv Funct Mater. 2025; 35(13):2417810. [22] LI J, LI L, WU T, et al. An Injectable Thermosensitive Hydrogel Containing Resveratrol and Dexamethasone-Loaded Carbonated Hydroxyapatite Microspheres for the Regeneration of Osteoporotic Bone Defects. Small Methods. 2024;8(1):e2300843. [23] DING Q, LIU W, ZHANG S, et al. Hydrogel loaded with thiolated chitosan modified taxifolin liposome promotes osteoblast proliferation and regulates Wnt signaling pathway to repair rat skull defects. Carbohydr Polym. 2024;336:122115. [24] CHATTERJEE S, HUI PC, SIU WS, et al. Influence of pH-responsive compounds synthesized from chitosan and hyaluronic acid on dual-responsive (pH/temperature) hydrogel drug delivery systems of Cortex Moutan. Int J Biol Macromol. 2021;168: 163-174. [25] WEI C, XING S, LI Y, et al. Gelatin/carboxymethyl chitosan/aloe juice hydrogels with skin-like endurance and quick recovery: Preparation, characterization, and properties. Int J Biol Macromol. 2024; 261(Pt 1):129720. [26] 徐达达.共价交联大黄多糖、淫羊藿多糖明胶水凝胶及其性能评价[D].兰州: 兰州理工大学,2019. [27] ZHANG Q, ZHANG M, WANG T, et al. Preparation of aloe polysaccharide/honey/PVA composite hydrogel: Antibacterial activity and promoting wound healing. Int J Biol Macromol. 2022;211:249-258. [28] 毕玉杰,马笃军,彭力平,等.中医药联合医用水凝胶治疗疾病的策略及意义[J].中国组织工程研究,2024,28(3):419-425. [29] 董心雨,董馨月,王婉婷,等.中药有效成分结合支架材料促进骨组织再生[J].中国组织工程研究,2024,28(20):3240-3245. [30] KIM JM, LIN C, STAVRE Z, et al. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells. 2020;9(9):2073. [31] LEENA RS, VAIRAMANI M, SELVAMURUGAN N. Alginate/Gelatin scaffolds incorporated with Silibinin-loaded Chitosan nanoparticles for bone formation in vitro. Colloids Surf B Biointerfaces. 2017;158:308-318. [32] YANG J, ZHANG L, WANG Y, et al. Dihydromyricetin-loaded oxidized polysaccharide/L-arginine chitosan adhesive hydrogel promotes bone regeneration by regulating PI3K/AKT signaling pathway and MAPK signaling pathway. Carbohydr Polym. 2024;346:122614. [33] LIU H, JIAO Y, FOROUZANFAR T, et al. High-strength double-network silk fibroin based hydrogel loaded with Icariin and BMSCs to inhibit osteoclasts and promote osteogenic differentiation to enhance bone repair. Biomater Adv. 2024;160:213856. [34] WEI B, WANG W, LIU X, et al. Gelatin methacrylate hydrogel scaffold carrying resveratrol-loaded solid lipid nanoparticles for enhancement of osteogenic differentiation of BMSCs and effective bone regeneration. Regen Biomater. 2021;8(5): rbab044. [35] YU Q, MENG Z, LIU Y, et al. Photocuring Hyaluronic Acid/Silk Fibroin Hydrogel Containing Curcumin Loaded CHITOSAN Nanoparticles for the Treatment of MG-63 Cells and ME3T3-E1 Cells. Polymers (Basel). 2021;13(14):2302. [36] AMIRYAGHOUBI N, FATHI M, SAFARY A, et al. In situ forming alginate/gelatin hydrogel scaffold through Schiff base reaction embedded with curcumin-loaded chitosan microspheres for bone tissue regeneration. Int J Biol Macromol. 2024;256(Pt 2):128335. [37] YU T, DING Q, WANG N, et al. Cranial repair-promoting effect of oxidised sodium alginate/amino gelatine injectable hydrogel loaded with deer antler blood peptides. Int J Biol Macromol. 2025;305(Pt 1):141116. [38] DONG M, YANG X, LU J, et al. Injectable rBMSCs-laden hydrogel microspheres loaded with naringin for osteomyelitis treatment. Biofabrication. 2023;15(4). doi: 10.1088/1758-5090/aceaaf. [39] CHEN Y, QIU Z, HU X, et al. Biofunctional supramolecular injectable hydrogel with spongy-like metal-organic coordination for effective repair of critical-sized calvarial defects. Asian J Pharm Sci. 2025; 20(1):100988. [40] 解强,常俊杰,高俊,等.阿仑膦酸修饰的水凝胶负载柚皮素骨靶向系统构建及其增强成骨分化作用 [J].中国骨质疏松杂志,2023,29(4):531-537. [41] 杨世超,宋慕格,李幸,等.淫羊藿苷修复骨缺损及其作用机制研究[J].中国骨质疏松杂志,2024,30(5):720-724,744. [42] KODAMA J, WILKINSON KJ, IWAMOTO M, et al. The role of hypertrophic chondrocytes in regulation of the cartilage-to-bone transition in fracture healing. Bone Rep. 2022;17:101616. [43] AN X, ZHOU F, LI G, et al. Cyaonoside A-loaded composite hydrogel microspheres to treat osteoarthritis by relieving chondrocyte inflammation. J Mater Chem B. 2024;12(17):4148-4161. [44] LIAO S, ZHOU K, KANG Y, et al. Enhanced cartilage repair using gelatin methacryloyl hydrogels combined with icariin and magnesium-doped bioactive glass. Artif Cells Nanomed Biotechnol. 2025;53(1): 181-193. [45] ZHU Y, YE L, CAI X, et al. Icariin-Loaded Hydrogel Regulates Bone Marrow Mesenchymal Stem Cell Chondrogenic Differentiation and Promotes Cartilage Repair in Osteoarthritis. Front Bioeng Biotechnol. 2022;10:755260. [46] LI S, YUAN Q, YANG M, et al. Enhanced cartilage regeneration by icariin and mesenchymal stem cell-derived extracellular vesicles combined in alginate-hyaluronic acid hydrogel. Nanomedicine. 2024;55:102723. [47] AN X, ZHOU Q, SHENG S, et al. Enhanced Chondrogenic Potential and Osteoarthritis Treatment Using Cyaonoside A-Induced MSC Delivered via a Hyaluronic Acid-Based Hydrogel System. Aging Dis. 2025. doi: 10.14336/AD.2024.10016. [48] JIANG W, XIANG X, SONG M, et al. An all-silk-derived bilayer hydrogel for osteochondral tissue engineering. Mater Today Bio. 2022; 17:100485. [49] WANG Z, LI K, SUN H, et al. Icariin promotes stable chondrogenic differentiation of bone marrow mesenchymal stem cells in self‑assembling peptide nanofiber hydrogel scaffolds. Mol Med Rep. 2018;17(6): 8237-8243. [50] VAN DER KRAAN PM. The Interaction between Joint Inflammation and Cartilage Repair. Tissue Eng Regen Med. 2019;16(4):327-334. [51] YANG Y, HU Q, SHAO Q, et al. A Baicalin-Based Functional Polymer in Dynamic Reversible Networks Alleviates Osteoarthritis by Cellular Interactions. Adv Sci (Weinh). 2025;12(10):e2410951. [52] LIU J, LI D, SUN X, et al. Icariine Restores LPS-Induced Bone Loss by Downregulating miR-34c Level. Inflammation. 2016;39(5):1764-1770. [53] 周晓洁,姚辛敏,周妍妍.淫羊藿的药理作用研究进展[J].中医药学报,2022, 50(11):112-125. [54] LI X, SUN Z, SHANG X, et al. Sequential delivery of IL-10 and icariin using nanoparticle/hydrogel hybrid system for prompting bone defect repair. Mater Today Bio. 2024;29:101374. [55] TANG M, WANG G, LI J, et al. Flavonoid extract from propolis alleviates periodontitis by boosting periodontium regeneration and inflammation resolution via regulating TLR4/MyD88/NF-κB and RANK/NF-κB pathway. J Ethnopharmacol. 2024;319(Pt 3):117324. [56] ZHENG Y, SUN RT, HAN MC, et al. Injectable Gypsogenin-Based Composite Hydrogel Enhances Osteoporotic Bone Regeneration by Alleviating Oxidative Injury via Promoting AMPKα Phosphorylation. Adv Funct Mater. 2025. doi: 10.1002/adfm.202424326. [57] SUN Q, YIN W, RU X, et al. Dual role of injectable curcumin-loaded microgels for efficient repair of osteoarthritic cartilage injury. Front Bioeng Biotechnol. 2022;10:994816. [58] HUANG R, HU C, XU S, et al. 3D-Printed Bifunctional Scaffold for Treatment of Critical Bone Defects Based on Osteoimmune Microenvironment Regulation and Osteogenetic Effects. ACS Appl Mater Interfaces. 2024;16(46):63345-63357. [59] SHAO B, FU Y, LI B, et al. Icariin-loaded chitosan/β-glycerophosphate thermosensitive hydrogel enhanced infection control and bone regeneration in canine with infectious bone defects. J Biomater Appl. 2025;39(7):696-713. [60] XU S, ZHAO S, JIAN Y, et al. Icariin-loaded hydrogel with concurrent chondrogenesis and anti-inflammatory properties for promoting cartilage regeneration in a large animal model. Front Cell Dev Biol. 2022;10:1011260. [61] ASGARI N, BAGHERI F, ESLAMINEJAD MB, et al. Dual functional construct containing kartogenin releasing microtissues and curcumin for cartilage regeneration. Stem Cell Res Ther. 2020;11(1):289. [62] STEGEN S, VAN GASTEL N, CARMELIET G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19-27. [63] FAN D, LIU H, ZHANG Z, et al. Resveratrol and Angiogenin-2 Combined With PEGDA/TCS Hydrogel for the Targeted Therapy of Hypoxic Bone Defects via Activation of the Autophagy Pathway. Front Pharmacol. 2021;12:618724. [64] WANG W, CHEN H, XIAO J, et al. Microenvironment-responsive injectable hydrogel for neuro-vascularized bone regeneration. Mater Today Bio. 2024;29: 101369. [65] YANG J, HAN Y, ZHANG L, et al. Taxifolin-loaded cellulose/l-arginine-chitosan hydrogel promoting bone defect repair through osteogenesis and angiogenesis. Int J Biol Macromol. 2024;283(Pt 3):137843. [66] DAYA R, XU C, NGUYEN NT, et al. Angiogenic Hyaluronic Acid Hydrogels with Curcumin-Coated Magnetic Nanoparticles for Tissue Repair. ACS Appl Mater Interfaces. 2022;14(9):11051-11067. |
| [1] | Liu Yang, Liu Donghui , Xu Lei, Zhan Xu, Sun Haobo, Kang Kai. Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2072-2080. |
| [2] | Guo Yuchao, Ni Qianwei, Yin Chen, Jigeer·Saiyilihan, Gao Zhan . Quaternized chitosan hemostatic materials: synthesis, mechanism, and application [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2091-2100. |
| [3] | Liu Dawei, Cui Yingying, Wang Fanghui, Wang Zixuan, Chen Yuhan, Li Yourui, Zhang Ronghe. Epigallocatechin gallate-mediated bidirectional regulation of reactive oxygen species and its application in nanomaterials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2101-2112. |
| [4] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [5] | Liu Hongjie, Mu Qiuju, Shen Yuxue, Liang Fei, Zhu Lili. Metal organic framework/carboxymethyl chitosan-oxidized sodium alginate/platelet-rich plasma hydrogel promotes healing of diabetic infected wounds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1929-1939. |
| [6] | Chen Weifei, Mei Yuandong, Ju Jihui. Repair of infected bone defect with dual-ion time-sequenced release multifunctional hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5188-5200. |
| [7] | Xu Yixuan, Yao Jun, Liu Xulu, Li Xinlian, Liu Zhixiong, Zhang Zhihong. Vancomycin-containing porcine skin acellular extracellular matrix hydrogel promotes wound healing in skin infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5214-5228. |
| [8] | Lin Kejian, Chai Yinghong, Zou Jie, Huang Ruixin, Fang Yongchao, Huang Jing, Yang Qin, Luo Xia, Zhang Hong. Preparation of Cu2+-containing microarc oxidation functional coating on medical magnesium alloy and its anti-tumor and angiogenesis-promoting effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5103-5114. |
| [9] | Zhou Xiaohui, Wang Siyi, Zhou Qiyun, He Zhao, Jia Yujuan, Wang Yuanbin, Ma Jianwu, Chen Gang, Zheng Feng, Chu Genglei. Nanohydroxyapatite-polyether carbonate urethane electrospinning membrane promotes bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5134-5142. |
| [10] | Cao Yuqing, Guo Meiling, Liu Feng, Wei Junchao. Preparation, classification and application of polysaccharide-based hydrogels in skin damage repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5257-5269. |
| [11] | Diao Youlu, Gao Jia, Pan Guoqing. Recruitable tissue repair biomaterials: advantages of regulating cell and factor migration and improving tissue integration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5270-5281. |
| [12] | Wang Liang, Zhang Xin, He Wei, Wang Jian. Clinical application and prospects of MXene-based materials for the repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5282-5294. |
| [13] | He Zhenzhen, Huang Hanji, Wang Jiawei, Xie Qingtiao, Jiang Xianfang. Role of bioscaffolds in the repair of inflammation-driven bone and cartilage destruction and structural damage in temporomandibular joint [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5312-5320. |
| [14] | Wang Zitong, Wu Zijian, Yang Aofei, Mao Tian, Fang Nan, Wang Zhigang. Biomaterials regulate microenvironment imbalance for treating spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5321-5330. |
| [15] | Yan Qiquan, Yang Libin, Li Mengjun, Ni Yazhuo, Chen Keying, Xu Bo, Li Yaoyang, Ma Shiqing, Li Rui, Li Jianwen. Preparation and antibacterial properties of porcine small intestinal submucosal composite nanohydroxyapatite bioscaffold loaded with antimicrobial peptide KR-12-a5 [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 384-394. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||