Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (14): 3709-3716.doi: 10.12307/2026.653
Previous Articles Next Articles
Characteristics and strategies of 3D-printed biomimetic bioceramic scaffolds for repairing jaw defects
Xiong Jiaying, Shen Jieyi, Lyu Jiahong
- School of Stomatology, Jinan University, Guangzhou 510632, Guangdong Province, China
-
Received:2025-06-09Accepted:2025-07-05Online:2026-05-18Published:2025-09-15 -
Contact:Lyu Jiahong, PhD, Lecturer, Attending physician, School of Stomatology, Jinan University, Guangzhou 510632, Guangdong Province, China -
About author:Xiong Jiaying, School of Stomatology, Jinan University, Guangzhou 510632, Guangdong Province, China -
Supported by:Science and Technology Projects in Guangzhou, No. 2023A04J1286 (LJH); Guangzhou Basic Research Plan City-School Joint Funding Special Project, No. 2025A03J3457 (LJH)
CLC Number:
Cite this article
Xiong Jiaying, Shen Jieyi, Lyu Jiahong. Characteristics and strategies of 3D-printed biomimetic bioceramic scaffolds for repairing jaw defects[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3709-3716.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
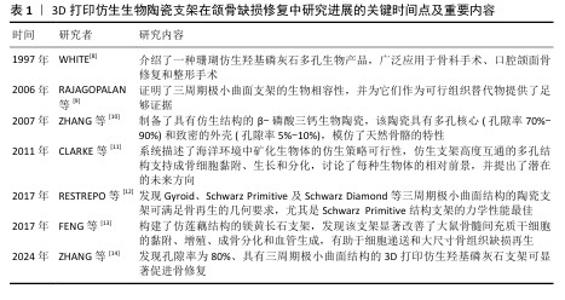
2.1 3D打印仿生生物陶瓷支架研究的里程碑 见表1。 2.2 颌骨缺损修复的特点、要求及策略 颌骨缺损通常具有解剖结构复杂、修复难度大等特点,同时缺损区解剖结构的破坏可直接导致咀嚼效能降低、吞咽协调障碍及语音共鸣异常,严重影响患者的生理功能与生活质量。颌骨缺损修复是一个动态过程,要求材料在初期提供足够的机械强度以维持缺损区形态,长期降解速率与骨再生速度匹配。颌骨缺损修复材料不仅需要能重建颌骨的解剖形态和力学支撑,恢复咀嚼、语音等生理功能,以恢复面部的对称性和自然外观,还要具有良好的生物相容性和骨整合能力。因此,用于颌骨缺损的仿生结构支架需具备精准符合缺陷、优良精度以及优异的生物学性能等特点[15]。 目前,颌骨缺损修复的基本策略包括天然骨移植和合成骨移植。自体骨移植仍是目前治疗颌骨缺损的金标准,其中腓骨瓣自体移植物已成为重建颌面缺损的常用选择,自体骨移植具有优异的骨生成能力,但存在供区损伤和骨量有限的问题[16]。异体骨移植避免了供区损伤的问题,但可能引发免疫排斥和感染风险。人工合成骨材料分为生物医用金属材料、生物医用高分子材料和生物医用无机非金属材料,其中金属材料存在生物活性差和应力屏蔽的缺点,天然高分子材料存在成本高昂的缺点,而人工合成高分子材料存在组织相容性差的缺点。尽管生物陶瓷材料存在力学脆性和降解动力学等问题,但具有良好的生物学性能,可借助3D打印技术定制个性化支架,展现出良好的应用前景[17]。另外,如何有效提升生物陶瓷支架的成骨活性与功能性,仍是当前骨组织工程领域亟待攻克的关键科学难题,核心挑战在于突破材料仿生设计。 生物陶瓷材料具有与骨骼的无机成分相似、刚度高、亲水性、生物活性、生物相容性、骨传导性和潜在的骨诱导性等特点,被广泛用于骨组织缺损的修复[18-19]。MA等[20]概述了3D打印生物陶瓷支架的特性为:可控且规则的多孔结构、足够的机械强度、生物相容性和生物降解性以及良好的生物活性,表明生物陶瓷材料支架可为骨再生构建稳定且良好的生长环境。 缺损部位具有复杂的形状,涉及与多种组织类型的界面。3D打印仿生生物陶瓷支架可通过个性化定制针对性构建出符合缺损形状并具有一定机械强度的支架材料,使它与周围面部骨骼的轮廓准确匹配。选择不同的3D打印方法可构建具有不同的孔隙率及降解速率的3D打印陶瓷支架材料,使支架与骨再生速率匹配,从而利于骨再生过程[21]。 在颌骨缺损修复过程中,血管生成和骨再生这2个过程通常是耦合的,这要求修复材料需具有良好的生物活性及引导血管内皮细胞、间充质干细胞迁入及诱导分化的能力[22]。支架材料可通过释放活性离子来增强成骨成血管功能,也可以在支架材料表面负载不同活性药物来增强成骨成血管功能,但期间可能引入杂质并影响支架的生物学效果[23]。对支架的结构进行改进也可以增强支架的生物活性。支架结构可模拟天然骨组织设计,将更有利于骨组织再生。也有研究受自然界中的多孔莲藕、海螺等生物结构启发,设计各种自然仿生结构的支架,增强支架的物质递送能力。目前随着3D打印技术的快速发展,具有仿生结构的生物陶瓷支架在诱导骨缺损区域血管生成和新骨生成中表现出越来越大的潜力。"

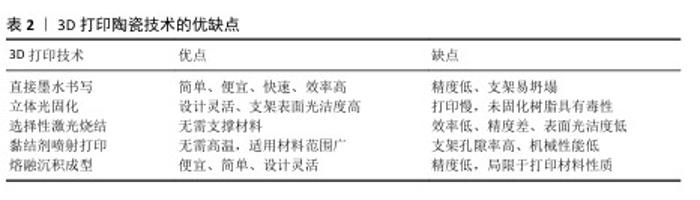
2.3 具有仿生结构的颌骨缺损修复材料的制备策略 2.3.1 生物陶瓷是3D打印颌骨缺损修复材料的常用选择 生物陶瓷分为生物惰性陶瓷和生物活性陶瓷,其中生物活性陶瓷一般具有优越的生物相容性、生物可降解性和骨传导性等优点。磷酸钙陶瓷是骨组织工程中最常用的生物活性陶瓷,包括羟基磷灰石、磷酸三钙、双相磷酸钙等。羟基磷灰石是目前应用最广泛的生物陶瓷材料之一,钙磷比与人体骨组织的无机成分相似,具有良好的生物相容性和骨传导性[24]。作为磷酸钙材料中结构最稳定、溶解性最低的物相之一,羟基磷灰石植入后降解缓慢,可能增加感染风险[20]。磷酸三钙包括α-磷酸三钙和β-磷酸三钙,稳定性均低于羟基磷灰石,具有良好的生物可降解性和骨传导性,能在体内逐渐降解并被新骨组织替代[25]。其中,β-磷酸三钙因具有可易调控的降解速率而被广泛研究[26]。 2.3.2 3D打印仿生生物陶瓷的制备技术与应用优势 3D打印可个性化定制尺寸构建出仿生陶瓷支架。3D打印陶瓷支架技术包括立体光固化、选择性激光烧结、熔融沉积成型、直接墨水书写以及黏结剂喷射打印。文章总结了不同制备方法用于颌骨缺损修复材料的优缺点,见表2。 直接墨水书写技术是通过挤出具有特定流变特性的墨水材料来构建三维结构的一种3D打印技术,如今已成为最通用的增材制造方法之一。直接墨水书写的优点为实现了更简单、更便宜、更快速的制造过程,使用这种技术可以打印大量复杂的陶瓷支架结构,包括致密的整体部件和多孔支架陶瓷基复合材料[27]。 立体光固化技术使用激光聚焦到陶瓷浆料(光敏树脂和陶瓷粉体按比例称取配制)表面,使材料逐层凝固,叠加构成三维实体。该技术对结构设计具有高度的灵活性,允许对孔隙率、孔隙形态、孔径和生物陶瓷支架的互连性进行高精度控制[28]。 选择性激光烧结技术是使用激光烧结混合陶瓷粉末材料,在激光照射下粉末材料黏合在一起形成坚固的结构。由于陶瓷材料的熔点高,常通过加入各种聚合物制作混合粉末材料来降低熔点,同时聚合物可在烧结过程中被去除,使支架最终呈现多孔结构,有利于骨组织向内生长,或负载其他分子材料实现支架的多功能化[29-30]。 黏结剂喷射打印技术通过在粉末床上逐层喷射黏合剂来固化粉末颗粒,通过后处理增强强度和稳定性。相比选择性激光烧结技术,黏结剂喷射打印无需在高温下进行,因此适用材料范围较广。近年来,该技术已被广泛应用于生物陶瓷支架的制作[31]。由于陶瓷支架的高孔隙率和较低的机械性能,通过添加其他物质可增强支架的机械性能。KE等[32]制备了一种新型3D打印氧化镁/氧化锌-磷酸三钙支架,该支架具有更高的抗压强度和生物反应,在骨修复和再生的临床应用中具有巨大潜力。 熔融沉积成型技术通过加热将热塑性材料熔化,由喷头挤出并逐层沉积在构建平台上,堆叠成三维实体结构。熔融沉积成型技术的成本低、操作简便、设计灵活,但成形精度低且局限于打印材料的性质[33]。为了提高陶瓷材料的可塑性、可成型性和生物活性,可以引入聚乳酸、聚乳酸-羟基乙酸共聚物和聚己内酯等可降解聚合物。BRUYAS等[34]发现通过加入不同高比例的聚己内酯成分会改善支架的表面特性、降解速率和机械性能,从而促进细胞的黏附、增殖和成骨分化。 "

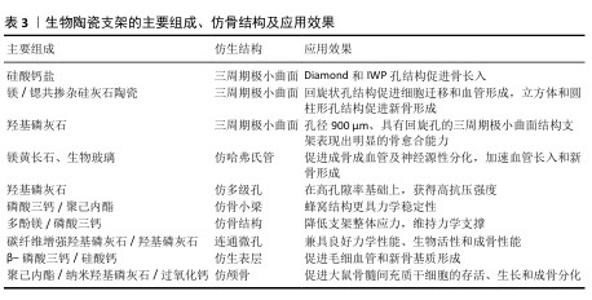
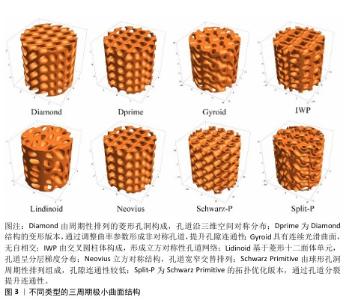
2.3.3 3D打印仿生生物陶瓷的仿骨结构设计 三周期极小曲面结构是一种平均曲率为零的周期性光滑隐式曲面,与松质骨结构相似,该结构设计包括多种类型,如Diamond、Dprime、Gyroid、IWP、Lindinoid、Neovius、Schwarz-P和Split-P等,见图3。不同结构之间的力学性能有差异,如制备的支架抗压强度大小顺序为:Diamond > IWP > Gyroid > Schwarz-P[35-36]。三周期极小曲面结构具有2个显著优点:可用数学函数精确表示其结构,通过调整功能参数可直接控制支架的基本性能,如孔隙率或体积比表面积等;表面光滑,相较于晶格结构没有锋利边缘或结点[37]。可通过调节不同的结构参数来调节三周期极小曲面的强度及孔隙率,以匹配不同密度和强度的骨组织。例如,SHEN等[38]制备了一系列具有精确孔隙几何形状的多孔硅酸盐生物陶瓷支架,证明Diamond和IWP孔几何形状有利于骨传导,促进骨组织的向内生长。有研究表明,孔径为300 μm的Split-P结构支架抗压强度高达152 MPa,满足皮质骨强度要求(100-200 MPa)[39]。Split-P结构支架也有利于细胞黏附、增殖和分化,显著提高成骨分化相关蛋白表达水平[40]。还有研究发现,不同大小孔径的Gyroid结构支架抗压强度与皮质骨相似,其中400 μm孔径支架表现出较高的机械强度和优异的骨再生能力[41]。可调节三周期极小曲面结构的孔形状来调控成骨成血管效果,有研究对比了回旋孔、圆柱孔和立方孔的三周期极小曲面支架发现,回旋状孔结构更利于人脐静脉内皮细胞的存活和增殖,并通过增强YAP/TAZ通路显著增强迁移和血管形成;立方体和圆柱形孔支架则在新骨形成方面表现出优异性能[42]。BOUAKAZ等[43]构建了孔径900 μm、具有回旋孔的三周期极小曲面结构支架,发现该结构支架可促进绵羊体内成骨。 除三周期极小曲面结构外,还有许多学者研究模仿骨结构支架以诱导血管长入和新骨生成。例如,ZHANG等[44]基于数字光处理技术构建了仿骨结构(如哈弗式管、伏克曼管)支架,该支架能在体外诱导成骨、血管生成和神经源性分化,在体内加速血管长入和新骨形成。有研究根据天然骨“骨髓腔-松质骨-皮质骨”的梯度结构,制备了分形仿生梯度骨组织工程支架[45]。还有研究通过仿哈弗式管和仿骨小管的分级微管结构与细晶结构构建了生物活性陶瓷支架,发现该支架能提高骨修复效果[46]。TANG等[47]通过两步冷冻铸造法制备了具有仿生骨多级孔结构的羟基磷灰石支架,该支架具有从内到外径向排列的孔隙率梯度。LI等[48]通过对椎骨状二维切片图像进行三维重建模仿制备了多孔支架,这些支架可通过优化控制渗透率调节细胞的黏附、迁移和分化微环境。LV等[49]对比具有矩形、三角形、蜂窝和菱形孔形状的磷酸三钙/聚己内酯复合支架,发现蜂窝结构支架更具力学稳定性。HU等[50]用低温成型方法构建表面负载多酚镁的α/β-磷酸三钙支架,仿生骨组织支架具有类似于天然骨的结构、组成及力学稳定性。ZHAO等[51]通过调整多孔和碳纤维增强羟基磷灰石的比例,制备了功能和结构分层的仿生生物陶瓷支架,发现该支架兼具优异的力学性能、生物活性及成骨性能。还有学者模仿类骨表面的微纳米晶体,在复合陶瓷(β-磷酸三钙/硅酸钙)表面构建了均匀的羟基磷灰石仿生表层,这有利于细胞的黏附,可增强骨髓间充质干细胞得成骨分化和人脐静脉内皮细胞基因表达,促进毛细血管和新骨基质形成[52]。有学者还模拟天然颅骨结构制备了一种类皮质骨-松质骨-皮质骨的仿生支架,并复合明胶-过氧化钙微球,该支架不仅具有良好的生物相容性、生物降解性和力学性能,还在缺氧条件下通过持续缓释氧气促进大鼠骨髓间充质干细胞的存活、生长和成骨分化[53]。 文章总结了生物陶瓷支架的各种组成、仿骨结构及效果,见表3。 "

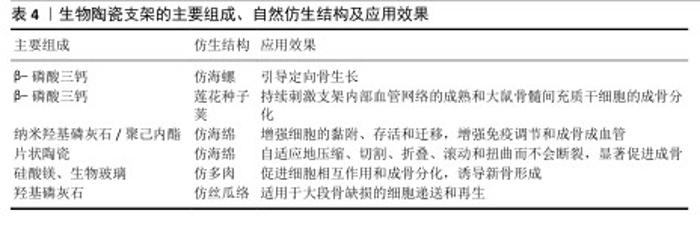
2.3.4 3D打印仿生生物陶瓷的自然仿生结构设计 新骨生成需构建通道使细胞与血管到达骨缺损部位,学者们为此聚焦于提高支架的物质递送能力。有研究制备了一种具有海螺状结构的β-磷酸三钙基生物陶瓷支架,用于引导定向骨骼生长[54],相比于传统支架,螺旋结构可有效诱导细胞从支架底部迁移到顶部,有效提高物质运输能力,从而提高支架的成骨性能。HAN等[55]受莲花种子荚结构启发,以3D打印支架为莲花“外壳”,将缺氧模拟复合物去铁胺-脂质体直接注入支架中,成功构建了一种构建内部血管化的仿莲花的3D打印多孔生物陶瓷支架,显著促进了骨缺损修复。LIAN等[56]构建了仿分层多孔海绵状结构的纳米羟基磷灰石/丙交酯-聚己内酯共聚物支架,发现该支架能改善间充质干细胞的旁分泌功能,上调免疫调节、血管生成和成骨因子表达。YANG等[57]通过3D打印制备的仿海绵针状柔性生物陶瓷基支架,可自适应地压缩、切割、折叠、滚动和扭曲而不会发生断裂,突破了传统生物陶瓷支架的瓶颈,显著增强了支架的成骨活性。WANG等[58]构建的仿多肉植物结构生物陶瓷支架,可通过调节细胞分布及其相互作用改善骨再生。还有研究发现丝瓜络类多孔结构有利于骨再生,丝瓜络外壁具有类似于低承重部分松质结构的网状结构;孔隙率79%-93%,可满足骨再生要求;具有高表面积,有利于细胞的黏附、增殖、分化和迁移[59]。该研究认为丝瓜是低密度、高孔隙率和低流动阻力的理想模型,极其适合骨再生,据此构建了不同孔隙率的仿丝瓜络结构支架,发现孔隙率为72.1%和73.2%的支架的骨修复效果突出。 文章总结了生物陶瓷支架的各种组成、自然仿生结构及效果,见表4。 "

2.4 3D打印仿生生物陶瓷支架的实际应用 导致颌面部骨缺损的疾病众多,例如创伤、肿瘤、牙周病、根尖周病、放射性坏死骨髓炎等。3D打印仿生生物陶瓷支架是大块骨缺损修复策略的理想选择。目前大多数相关研究更多关注于支架的组分优化,常常在3D打印陶瓷支架中掺加各种生物活性离子或引入生物因子/物质,以提升修复效果[60-61]。例如,有研究制备了一种多离子型的生物活性硅酸镁锶多孔陶瓷支架[62];还有研究开发了一种蚕丝蛋白/羟基磷灰石的3D打印支架,并负载骨形态发生蛋白 2、血管内皮生长因子、神经生长因子,以提高成骨成血管性能[63]。 目前,3D打印仿生结构陶瓷支架应用于颌面部骨缺损修复的研究开始逐渐出现。例如,WU等[64]基于Voronoi-Tessellation原理构建了一种具有梯度孔结构的仿生颌面小梁修复体,该支架内部的梯度多孔结构为细胞黏附与增殖提供了良好的微环境,极大促进骨再生,通过模拟准静态双侧磨牙咬合发现该颌面修复体可有效平衡双侧颌面骨应力,证明了它有助于恢复患者口腔的正常功能;也有研究设计了具有梯度多孔结构的植入物用于颌面部骨缺损修复[65]。但目前关于3D打印仿生结构陶瓷支架的应用研究仍较少,多停留在基础实验阶段[66]。其中,汪焰恩等[66]在人工骨支架仿生结构优化设计和新型生物黏结剂的开发研制等研究中的成果在西安博恩生物科技有限公司成功转化。尽管如此,未来还需要在更多的临床试验中验证3D打印仿生陶瓷支架的安全性及有效性。未来,4D打印为构建动态、个性化和精确的骨组织工程支架提供了更大的潜力,因此一直被视为下一代骨骼修复技术[67]。4D打印在3D结构的基础上增加了一个维度(时间),用于骨再生的3个目的一般为形状记忆、药物输送和形状变形[68]。未来可通过光热治疗促进活性离子的释放,从而增强抗菌活性、调节局部微环境炎症反应;还可通过调节氧化应激微环境清除活性氧,以促进局部骨再生以及构建可控药物释放系统等,实现个性化、全周期骨再生。尽管仿生生物陶瓷支架面临长期安全性验证与产业化成本挑战,并且还需要通过提高生物制造和支架生产的速度来治疗大规模缺陷和潜在的大量患者,但未来与4D打印、计算机辅助技术的深度融合将推动3D打印仿生生物陶瓷支架颌骨修复的快速发展。 "

| [1] WU AM, BISIGNANO C, JAMES SL, et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021; 2(9):E580-E92. [2] KäMMERER PW, AL-NAWAS B. Bone reconstruction of extensive maxillomandibular defects in adults. Periodontol 2000. 2023;93(1):340-357. [3] BOSE S, AKDOGAN EK, BALLA VK, et al. 3D printing of ceramics: Advantages, challenges, applications, and perspectives. J Am Ceram Soc. 2024;107(12):7879-920. [4] 胡堃,王峻东,杨桂娟,等.3D打印智能仿生材料研究进展[J].数字印刷,2020(5): 1-15. [5] GUO W, LI B W, LI P, et al. Review on vat photopolymerization additive manufacturing of bioactive ceramic bone scaffolds. J Mater Chem B. 2023;11(40): 9572-9596. [6] KALIDINDI S. The role of three-dimensional (3D) printing in plastic and reconstructive surgery: innovations and applications. Eur J Plast Surg. 2024;47(1):96. [7] CHEN B, CHEN Q, ZHANG HD, et al. 3D-Printed Dual-Bionic Scaffolds to Promote Osteoconductivity and Angiogenesis for Large Segment Bone Restoration. Adv Funct Mater. 2024;35:2422691. [8] WHITE E. Biomaterials innovation: it’sa long road to the operating room. Mater Res Innovations. 1997;1(1):57-63. [9] RAJAGOPALAN S, ROBB RA. Schwarz meets Schwann: design and fabrication of biomorphic and durataxic tissue engineering scaffolds. Med Image Anal. 2006;10(5): 693-712. [10] ZHANG F, CHANG J, LU J, et al. Bioinspired structure of bioceramics for bone regeneration in load-bearing sites. Acta Biomater. 2007;3(6):896-904. [11] CLARKE S, WALSH P, MAGGS C, et al. Designs from the deep: Marine organisms for bone tissue engineering. Biotechnol Adv. 2011;29(6):610-617. [12] RESTREPO S, OCAMPO S, RAMíREZ J, et al. Mechanical properties of ceramic structures based on Triply Periodic Minimal Surface (TPMS) processed by 3D printing. J Phys Conf Ser. 2017; 935:012036. [13] FENG C, ZHANG W, DENG C, et al. 3D Printing of Lotus Root-Like Biomimetic Materials for Cell Delivery and Tissue Regeneration. Adv Sci (Weinh). 2017;4(12): 1700401. [14] ZHANG P, ZHOU Q, HE R. Three-Dimensionally Printed Bionic Hydroxyapatite (HAp) Ceramic Scaffolds with Different Structures and Porosities: Strength, Biocompatibility, and Biomedical Application Potential. Materials (Basel). 2024;17(24):6092. [15] NYBERG EL, FARRIS AL, HUNG B, et al. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann Biomed Eng. 2017; 45(1):45-57. [16] MIGLIORINI F, LA PADULA G, TORSIELLO E, et al. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: a comprehensive review of the literature. Eur J Med Res. 2021;26(1): 1-10. [17] JIANG XQ. Biomaterials for bone defect repair and bone regeneration.Zhonghua Kou Qiang Yi Xue Za Zhi. 2017;52(10): 600-604. [18] LIN H, ZHANG LY, ZHANG QY, et al. Mechanism and application of 3D-printed degradable bioceramic scaffolds for bone repair. Biomater Sci. 2023;11(21): 7034-7050. [19] DU M, CHEN J, LIU K, et al. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Composites. Part B. 2021;215:108790. [20] MA HS, FENG C, CHANG J, et al. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018;79:37-59. [21] SHEN C, WANG MM, WITEK L, et al. Transforming the Degradation Rate of β-tricalcium Phosphate Bone Replacement Using 3-Dimensional Printing. Ann Plast Surg. 2021;87(6):e153-e162. [22] JANG HJ, YOON JK. The Role of Vasculature and Angiogenic Strategies in Bone Regeneration. Biomimetics (Basel). 2024; 9(2):75. [23] SHAHROUZIFAR MR, SALAHINEJAD E, SHARIFI E. Co-incorporation of strontium and fluorine into diopside scaffolds: Bioactivity, biodegradation and cytocompatibility evaluations. Mater Sci Eng C Mater Biol Appl. 2019;103:109752. [24] 牛建华,易敏,许卫星.3D打印技术制备β-TCP仿生骨支架的形态结构特点及其成骨性能分析[J]. 口腔材料器械杂志,2023, 32(3):183-188. [25] JEONG J, KIM JH, SHIM JH, et al. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23(1):4. [26] GUVENDIREN M, MOLDE J, SOARES RMD, et al. Designing Biomaterials for 3D Printing. ACS Biomater Sci Eng. 2016;2(10):1679-1693. [27] SAADI M, MAGUIRE A, POTTACKAL NT, et al. Direct Ink Writing: A 3D Printing Technology for Diverse Materials. Adv Mater. 2022;34(28):2108855. [28] ZHANG YH, ZHANG Q, HE FP, et al. Fabrication of cancellous-bone-mimicking β-tricalcium phosphate bioceramic scaffolds with tunable architecture and mechanical strength by stereolithography 3D printing. J Eur Ceram Soc. 2022;42(14):6713-6720. [29] KAMBOJ N, KAZANTSEVA J, RAHMANI R, et al. Selective laser sintered bio-inspired silicon-wollastonite scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2020;116:1112223. [30] ZENG H, PATHAK JL, SHI YH, et al. Indirect selective laser sintering-printed microporous biphasic calcium phosphate scaffold promotes endogenous bone regeneration via activation of ERK1/2 signaling. Biofabrication. 2020;12(2): 025032. [31] ZHANG F, LI ZA, XU MJ, et al. A review of 3D printed porous ceramics. J Eur Ceram Soc. 2022;42(8):3351-3373. [32] KE DX, BOSE S. Effects of pore distribution and chemistry on physical, mechanical, and biological properties of tricalcium phosphate scaffolds by binder-jet 3D printing. Addit Manuf. 2018;22:111-117. [33] KUMAR P, SHAMIM, MUZTABA M, et al. Fused Deposition Modeling 3D-Printed Scaffolds for Bone Tissue Engineering Applications: A Review. Ann Biomed Eng. 2024;52(5):1184-1194. [34] BRUYAS A, LOU F, STAHL M, et al. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J Mater Res. 2018;33(14):1948-1959. [35] KOLAN KC, LEU MC, HILMAS GE, et al. Effect of material, process parameters, and simulated body fluids on mechanical properties of 13-93 bioactive glass porous constructs made by selective laser sintering. J Mech Behav Biomed Mater. 2012;13:14-24. [36] 刘思达.基于数字化光处理3D打印制备仿生结构磷酸三钙骨组织工程支架及其性能表征[D].北京:北京工业大学,2020. [37] FENG JW, FU JZ, YAO XH, et al. Triply periodic minimal surface (TPMS) porous structures: from multi-scale design, precise additive manufacturing to multidisciplinary applications. Int J Extreme Manuf. 2022; 4(2):022001. [38] SHEN MD, LI YF, LU FL, et al. Bioceramic scaffolds with triply periodic minimal surface architectures guide early-stage bone regeneration. Bioact Mater. 2023;25: 374-386. [39] ZHANG Q, MA LM, JI XF, et al. High-Strength Hydroxyapatite Scaffolds with Minimal Surface Macrostructures for Load-Bearing Bone Regeneration. Adv Funct Mater. 2022; 32(33):2204182. [40] 崔越.3D打印高强度三周期极小曲面羟基磷灰石支架用于骨修复的研究[D].广州:华南理工大学,2021. [41] 李梦.光固化3D打印仿生生物活性陶瓷骨修复支架研究[D].武汉:武汉理工大学,2022. [42] LI YF, LI JF, JIANG S, et al. The design of strut/TPMS-based pore geometries in bioceramic scaffolds guiding osteogenesis and angiogenesis in bone regeneration. Mater Today Bio. 2023;20:100667. [43] BOUAKAZ I, DROUET C, GROSSIN D, et al. Hydroxyapatite 3D-printed scaffolds with Gyroid-Triply periodic minimal surface (TPMS) porous structure: Fabrication and an<i> in</i><i> vivo</i> pilot study in sheep. Acta Biomater. 2023;170:580-895. [44] ZHANG M, LIN R, WANG X, et al. 3D printing of Haversian bone-mimicking scaffolds for multicellular delivery in bone regeneration. Sci Adv. 2020;6(12):eaaz6725. [45] 屈华伟.挤出式3D打印仿生梯度组织工程支架的设计与制备研究[D].哈尔滨:哈尔滨工业大学,2022. [46] 徐丹蕾.磷酸钙生物活性陶瓷微尺度调控与骨修复性能研究[D].广州:华南理工大学,2023. [47] TANG YF, ZHAO K, HU L, et al. Two-step freeze casting fabrication of hydroxyapatite porous scaffolds with bionic bone graded structure. Ceram Int. 2013;39(8): 9703-9707. [48] LI X, WANG Y, ZHANG B, et al. The design and evaluation of bionic porous bone scaffolds in fluid flow characteristics and mechanical properties. Comput Methods Programs Biomed. 2022;225:107059. [49] LV X, WANG S, XU Z, et al. Structural Mechanical Properties of 3D Printing Biomimetic Bone Replacement Materials. Biomimetics (Basel, Switzerland). 2023; 8(2):166. [50] HU X, CHEN J, YANG S, et al. 3D Printed Multifunctional Biomimetic Bone Scaffold Combined with TP-Mg Nanoparticles for the Infectious Bone Defects Repair. Small (Weinheim an der Bergstrasse, Germany). 2024;20(40):e2403681. [51] ZHAO XN, FAN Q, CHEN XY, et al. One-step preparation of functionally hierarchical and structurally hierarchical biomimetic bioceramics composed of porous hydroxyapatite and carbon fiber reinforced hydroxyapatite composites. Mater Chem Phys. 2022;283. doi:10.1016/j.matchemphys.2022.126012. [52] LIU X, MIAO Y, LIANG H, et al. 3D-printed bioactive ceramic scaffolds with biomimetic micro/nano-HAp surfaces mediated cell fate and promoted bone augmentation of the bone-implant interface in vivo. Bioact Mater. 2022;12:120-132. [53] WANG Y H, XIE C N, ZHANG Z M, et al. 3D Printed Integrated Bionic Oxygenated Scaffold for Bone Regeneration. ACS Appl Mater Interfaces. 2022;14(16): 29506-29520. [54] FENG BS, ZHANG M, QIN C, et al. 3D printing of conch-like scaffolds for guiding cell migration and directional bone growth. Bioact Mater. 2023;22:127-140. [55] HAN X, SUN M, CHEN B, et al. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioact Mater. 2021;6(6):1639-1652. [56] LIAN M, SUN B, HAN Y, et al. A low-temperature-printed hierarchical porous sponge-like scaffold that promotes cell-material interaction and modulates paracrine activity of MSCs for vascularized bone regeneration. Biomaterials. 2021; 274:120841. [57] YANG Z, XUE J, LI T, et al. 3D printing of sponge spicules-inspired flexible bioceramic-based scaffolds. Biofabrication.2022;14(3):035009. [58] WANG Y, WANG Z, YU X, et al. 3D-Printing of succulent plant-like scaffolds with beneficial cell microenvironments for bone regeneration. J Mater Chem B. 2023; 11(24):5523-5536. [59] CHEN QH, ZOU B, LAI QG, et al. 3D printing and osteogenesis of loofah-like hydroxyapatite bone scaffolds. Ceram Int. 2021;47(14):20352-20361. [60] 景卓荦.3D打印多功能生物活性陶瓷支架研究[D].杭州:杭州电子科技大学, 2023. [61] 王文晓.面向骨组织工程的PLA/HA/Mg复合支架3D成形及其性能研究[D].青岛:青岛大学,2023. [62] GAN JX, LI ZK, XIONG JY, et al. Fabrication, physicochemical properties, and cytocompatibility of 3D-printed Sr<sub>2</sub>MgSi<sub>2</sub>O<sub>7</sub> bioceramic scaffolds calcined at different temperature for bone repair. J Eur Ceram Soc. 2025;45(1):116856. [63] FITZPATRICK V, MARTíN-MOLDES Z, DECK A, et al. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials. 2021; 276:120995. [64] WU HD, CHAO L, YI YJ, et al. Design and 3D printing of integrated bionic porous ceramic maxillofacial prosthesis. J Mater Sci. 2022;57(43):20366-20379. [65] PENG WM, LIU YF, JIANG XF, et al. Bionic mechanical design and 3D printing of novel porous Ti6Al4V implants for biomedical applications. J Zhejiang Univ Sci B. 2019; 20(8):647-659. [66] 汪焰恩,魏庆华,张娟,等.可发育陶瓷仿生骨精确制造技术及应用[R].西安:西北工业大学,2021. [67] CHEN X, HAN S, WU W, et al. Harnessing 4D printing bioscaffolds for advanced orthopedics. Small Adv Healthcare Mater. 2022;18(36):2106824. [68] GHAREHDAGHI N, NOKHBATOLFOGHAHAEI H, KHOJASTEH A. 4D printing of smart scaffolds for bone regeneration: a systematic review. Biomed Mater. 2025;20(1):012003. |
| [1] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [2] | Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970. |
| [3] | Bai Xiangyu, Huo Feng, Hao Yan, Wang Zecheng, Guo Xiaoyu. Platelet-derived growth factor BB-loaded chitosan/reduced graphene oxide scaffold for repairing alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 329-337. |
| [4] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [5] | Shi Xiaonan, Wu Xuan, Zhang Daxu, Hu Jingjing, Zheng Yazhe, Liu Yutong, Zhao Shuo, Li Weilong, Ye Shujun, Wang Jingyi, Yan Li. Preparation and characterization of 3D printed microstructured silk fibroin scaffold for liver injury repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3618-3625. |
| [6] | Gong Yukang, Ye Gaoqi, Wang Chenhao, Chen Dejin, Gao Wenshan. Effects and mechanisms of natural polyphenol-based hydrogels in promoting bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3675-3686. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||