Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (5): 771-776.doi: 10.12307/2024.265
Previous Articles Next Articles
Effect of micro-arc oxidation treatment on biological activity of medical metals
Wang Yeyuan1, Du Yilang1, Yu Dehao1, Ning Fengting1, Bai Bing2
- 1School of Stomatology, China Medical University, Shenyang 110001, Liaoning Province, China; 2Second Department of Prosthodontics, Hospital of Stomatology, China Medical University, Liaoning Institute of Stomatology, Shenyang 110001, Liaoning Province, China
-
Received:2023-03-20Accepted:2023-04-26Online:2024-02-18Published:2023-08-17 -
Contact:Bai Bing, Associate professor, Associate chief physician, Second Department of Prosthodontics, Hospital of Stomatology, China Medical University, Liaoning Institute of Stomatology, Shenyang 110001, Liaoning Province, China -
About author:Wang Yeyuan, School of Stomatology, China Medical University, Shenyang 110001, Liaoning Province, China Du Yilang, School of Stomatology, China Medical University, Shenyang 110001, Liaoning Province, China -
Supported by:Northeastern University-China Medical University National College Student Innovation Program, No. 220268 (to BB)
CLC Number:
Cite this article
Wang Yeyuan, Du Yilang, Yu Dehao, Ning Fengting, Bai Bing. Effect of micro-arc oxidation treatment on biological activity of medical metals[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 771-776.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
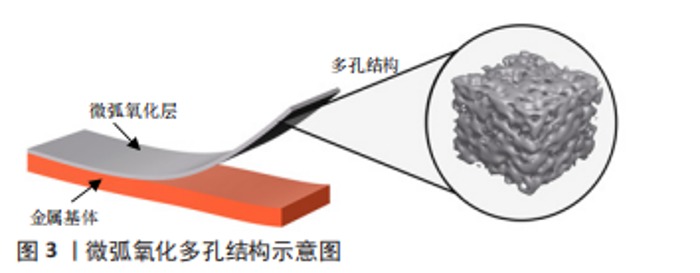
2.1 改善材料表面理化和生物学性能 2.1.1 表面形貌 表面形貌会影响材料表面的亲水性和粗糙度[3]。材料表面亲水性可以影响表面蛋白的黏附,通过吸附不同的蛋白质构建细胞外基质,进而介导细胞行为。LIN等[4]发现纳米级微弧氧化表面可以增加材料表面的亲水性,提供更多结合位点,利于纤维连接蛋白和牛血清白蛋白的黏附。亲水性也与细胞黏附相关,有研究发现超疏水涂层的生物活性差,而一步法微弧氧化可增强材料的亲水性和表面粗糙度,能够使材料表现出更强的生物活性[3,5]。 经微弧氧化处理的表面可形成相互连通的孔隙,达到的微米级粗糙度会增加细胞外基质的吸附,并改善骨组织和植入物之间的机械互锁。微弧氧化处理可以在多孔钛支架表面形成均匀致密并具有生物相容性的涂层,有利于细胞的黏附、增殖和分化,并且在体内表现出更好的骨结合和骨组织长入,能够维持小鼠成骨细胞的高水平增殖[6-7]。与抛光处理组相比,在3-10 μm的孔隙范围内,钛支架孔隙越大越有利于骨髓间充质干细胞的增殖、黏附和成骨分化,并可以促进人骨肉瘤细胞系(MG63)Ⅰ型胶原蛋白α1链、骨形态发生蛋白2和骨钙素等成骨基因的表达[8-9]。ZHANG等[10]研究不同表面粗糙度的钛表面,发现不同的粗糙度会影响破骨细胞的生成,间接调节骨结合,在粗糙度为1-1.5 μm时骨结合效果最佳,这也支持了其他研究微弧氧化微米级表面粗糙度可以提高生物活性的结果。图3为微弧氧化的多孔结构示意图。"
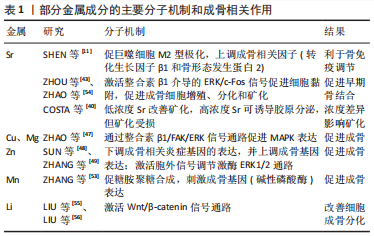
此外还有研究表明,表面形貌可影响巨噬细胞的分化[11-15]。高亲水性和适宜粗糙度的钛表面可诱导巨噬细胞向M2型极化,从而创造了一个可以减少愈合时间和增强骨结合的微环境。M2型巨噬细胞分泌的白细胞介素4、白细胞介素10、白细胞介素13等抗炎因子和骨形态发生蛋白2、转化生长因子β等生长因子,一方面能够显著刺激间充质干细胞/前体成骨细胞成骨分化并促进骨矿化;另一方面可以抑制破骨细胞前体的分化和成熟破骨细胞的活性,从而抑制骨吸收[12-13,16]。M2型巨噬细胞分泌的血小板衍生生长因子BB能够有效促进新生血管的形成,从而加速骨修复进程[15]。也有学者指出,在微弧氧化钛涂层表面上的巨噬细胞也可以向M1表型极化,通过炎症因子如肿瘤坏死因子α、白细胞介素6和白细胞介素1β的表达和释放,巨噬细胞可以在种植体周围形成炎症微环境,促进成骨样细胞的胶原合成和基质矿化,促进了植入物植入后的成骨作用[17]。 微弧氧化在心血管支架领域也有广泛的应用前景。一个优秀的血管支架应该具备良好的生物相容性,可以使动脉壁恢复正常,减少血栓、血管再狭窄等风险[18-19]。微弧氧化涂层原位生长的二氧化钛由于具有诱导快速再内皮化、抗血栓作用、良好的耐腐蚀性和良好的生物相容性等优点,被广泛用于支架表面改性[18],例如:利用层状双氢氧化物封闭微弧氧化涂层孔隙可以使微弧氧化涂层进一步促进内皮细胞的黏附、增殖和迁移[20]。然而有体外实验指出,同一涂层对不同细胞表现出不同的细胞毒性,在镁基支架表面经微弧氧化处理形成的MgO/Mg(OH)2,虽然能够促进有修复功能的脂肪组织来源间充质干细胞的黏附增殖,但对较为敏感脆弱的人脐静脉内皮细胞和平滑肌细胞具有较大的细胞毒性[19]。若能降低涂层的细胞毒性,微弧氧化处理后的心血管支架的应用价值将会显著提高。 2.1.2 弹性模量 对于骨内植入物而言,材料与周围骨组织弹性模量的匹配是成功植入的重要因素之一[21]。纯钛的弹性模量约为107 GPa,钛合金如Ti-6Al-4V的弹性模量约为117 GPa,而骨皮质的弹性模量约为20 GPa,由于这些植入材料与骨组织的弹性模量不匹配,会产生应力屏蔽效应,从而导致骨吸收等不良影响[22]。CHEN等[23]发现在纯钛的微弧氧化涂层中嵌入二氧化硅纳米颗粒可以将表面弹性模量从93 GPa下降到6.7 GPa,与其他组相比,掺入二氧化硅纳米颗粒的微弧氧化层还表现出自噬体的高表达、碱性磷酸酶活性和矿化能力的高表达,这是由于二氧化硅纳米颗粒的微弧氧化层显著刺激了机械敏感性转录因子Yes相关蛋白和具有PDZ结合序列的转录共激活因子(transcriptional co-activator with PDZ-binding motif,TAZ)的表达水平和核定位。Yes相关蛋白/TAZ在调节干细胞分化潜能、增殖、分化方向和细胞代谢中发挥重要作用,细胞外基质和材料表面的弹性模量都可以影响Yes相关蛋白/TAZ的活性,研究者可以通过Yes相关蛋白/TAZ的表达量来检测生物材料的机械性能对细胞的影响程度。YAP/TAZ还可进一步抑制间充质干细胞中Akt和mTOR蛋白的磷酸化,导致自噬标志物蛋白表达升高,促进间充质干细胞的成骨分化,这一结果也证明了钛表面纳米结构可以通过Yes相关蛋白/β-连环蛋白-自噬信号通路促进成骨,从而控制细胞的存活和增殖[23-26]。 2.1.3 耐腐蚀性 体内生物电解质溶液的腐蚀性是金属植入物能否长期稳定行使功能的威胁之一。镁合金具有熔点低、可降解等优点,受到越来越多的关注,现阶段众多研究集中在如何调节镁合金在体内的降解速率上[27],镁合金降解时析出的氢气与随之产生的高pH值环境会影响周围组织[28]。减缓金属植入物的降解可为成骨活动提供充足时间。 应用微弧氧化技术可以多方面调节金属材料的耐腐蚀性[3,29-32]:一方面,金属腐蚀析出的氢气可以进入基体导致氢脆效应,进而影响植入体的机械性能,研究证明微弧氧化涂层不仅直接抑制了氢气进入基体,且其诱导的应力为压应力,也抑制了氢脆效应[33];另一方面,微弧氧化涂层在制备过程中会产生许多通向基体的孔隙和裂纹,这些孔隙为腐蚀介质提供了通道,对基体耐腐蚀性有负面影响。许多研究通过添加有效物质制备复合涂层来封闭孔隙,以进一步提高耐腐蚀性。LI等[34]采用水浴法在镁合金AZ31的微弧氧化表面制备Mg(OH)2并加入EDTA-2Na,可以封闭微弧氧化涂层的裂纹和孔隙,提高镁合金的耐腐蚀性。CHEN等[35]加入Cu可以获得稳定涂层,提高了镀层的耐腐蚀性并可以促进涂层表面钙磷沉积。有研究在微弧氧化涂层表面沉积碳膜如类金刚石和类石墨碳薄膜,以增加材料的耐腐蚀性[36],这些物质不仅能够增加材料的耐腐蚀性,还可以通过机械互锁和化学反应增加基体和微弧氧化涂层的结合力。 心血管支架的耐腐蚀性也很重要,支架材料需要良好的耐腐蚀性且降解均匀稳定,否则可能导致材料断裂或塌陷,而微弧氧化处理可以控制降解速率,从而达到要求。有研究指出,镁铝层状双氢氧化物通过封闭微弧氧化涂层孔隙增强耐腐蚀性,但是耐磨性差、易脱落[37]。 除耐腐蚀性以外,植入物在体内的磨损也是需要考虑的,耐磨性好则植入物的使用寿命长。有研究指出与2A50铝合金未处理的平整表面相比,铝合金表面制备的硬质陶瓷微弧氧化涂层经过钢球60 min磨耗仍未被磨穿,且在阴极电压-100 V的情况下表现出最佳耐磨性[38]。但另一方面,微弧氧化涂层的多孔表面形貌会导致涂层的磨损增加,有学者指出可采用旋涂法在硬质陶瓷表面制备热固性聚酰亚胺复合自润滑涂层,这种方法可以将润滑性能良好的涂层涂布在不规则的材料表面以减少摩擦[31]。综上,微弧氧化涂层可以显著提高材料的耐磨性。 2.2 通过微弧氧化技术融入生物活性物质 2.2.1 促成骨物质 (1)金属物质:锶(Sr)元素因可以降低结晶度、提高羟基磷灰石的生物降解性和诱导成骨潜能而被广泛研究[39]。通过对人成骨样细胞的体外研究发现,较低浓度的Sr可改善植入物的周围矿化,不会影响人成骨样细胞的初始黏附和活性,而较高浓度的Sr可诱导胶原分泌但阻碍矿化,这可能是因为矿化过程的延迟或诱导过程中缺乏羟基磷灰石的沉淀,故在涂层中加入低浓度的Sr可制备理想的骨植入材料[40]。涂层中释放的Sr离子能上调过氧化氢酶/超氧化物歧化酶活性,从而清除过量的氧自由基。此外有学者指出,在具有高密度Sr涂层上的巨噬细胞向M2型极化并表达多种成骨相关因子(转化生长因子β1和骨形态发生蛋白2),这一过程有利于骨组织的免疫调节和早期骨结合[11]。通过一步微弧氧化制备的含Ca、Sr的多层多孔TiO2涂层,由于Ca、Sr分布在外层涂层而更易被释放,提高了人骨髓间充质干细胞的黏附和铺展性能[41]。同样是一步制备,有学者在氧化涂层中同时加入Ag和Sr,使之获得较强的抗菌效能和成骨作用[42]。在含Ca、P、Co和F的TiO2涂层基础上,用微弧氧化技术加入的Sr,不仅不会改变原涂层的促血管生成能力和抑菌能力,而且可促进间充质干细胞的增殖、成骨分化和种植体的骨结合(Sr的含量在11%最为适宜)[43]。 LIANG等[44]通过微弧氧化技术在镁合金表面制备了Cu涂层,在具有良好抗菌性的同时,也能促成骨和促血管生成。Cu的加入亦可以促进骨髓间充质干细胞增殖,进而促进成骨分化[45]。但SONG等[46]指出过高的Cu含量虽然可以增加抗菌性但会抑制细胞增殖,如含质量分数0.24%Cu的涂层能促进细胞增殖,而含质量分数0.69% Cu的涂层则抑制细胞早期增殖活性。微弧氧化法在钛表面制备镁-铜-氟(Mg-Cu-F)共掺杂的TiO2微孔涂层无明显的细胞毒性,同时在体外能促进小鼠成骨细胞的黏附、增殖、分化、矿化和凋亡,研究表明该机制可能是由于促进MAPK表达并通过整合素β1/FAK/ERK信号通路来促进成骨[47]。 锌(Zn)可以通过微弧氧化技术均匀地结合在钛表面并稳定释放Zn离子,一方面下调骨髓间充质干细胞成骨周围环境中炎症基因的表达;另一方面上调成骨基因如骨桥蛋白、Ⅰ型胶原和骨钙素的表达,并且Zn离子还可激活细胞外信号调节激酶ERK1/2通路促进成骨分化,证明该涂层具有良好的成骨性和抗炎潜力[48-49]。 通过微弧氧化技术在钛合金表面加入适当的镁(质量分数2.97%),在促进骨髓间充质干细胞的黏附、增殖和成骨分化中具有优势[50-51]。在微弧氧化层中加入具有抗菌作用的Ga2O3和具有出色成骨生物活性的钽微粒,可诱导大鼠骨髓间充质干细胞的增殖和成骨分化[52]。锰元素的加入可以促进骨结构中糖胺聚糖的合成,并刺激成骨细胞中相关基因(如碱性磷酸酶)的表达,从而促进骨形成过程,避免骨吸收。植入Mn后的涂层对比纯钛涂层具有较大的接触角(122°)以及适当的微粗糙度(1.5 μm),有利于小鼠成骨细胞的黏附和羟基磷灰石晶体的沉积[53]。通过微弧氧化技术在钛表面制备Mn-TiO2微孔涂层,体外实验表明它可以提高小鼠成骨细胞的黏附、增殖、分化和矿化能力,体内实验表明其可以促进早期阶段的骨结合。上述过程可能是通过激活整合素β1介导的ERK/c-Fos信号通路来改变细胞的跨膜蛋白,使细胞更容易与吸附在涂层表面的血浆蛋白结合,提高涂层的黏附能力[54]。通过微弧氧化技术将锂(Li)加入到与骨松质结构相似的多孔缠绕钛丝支架上,可以通过激活Wnt/β-catenin信号通路来改善细胞的成骨分化[55]。体内体外实验均证明,以微弧氧化技术制备的含Li纳米多孔涂层可提高AZ91镁合金的耐腐蚀性、并促进血管生成和骨结合,这种成骨能力也可能是由于Li离子激活Wnt/β-catenin通路而实现的[56]。表1为上述部分金属成分的主要分子机制和成骨相关作用。"

(2)非金属物质:硅元素可能引起细胞周围微环境碱化,并激活钙通道增加钙离子内流,从而促进细胞在涂层表面早期黏附和伪足形成;硅还能与氧结合形成具有三维网络结构的硅酸盐,吸附游离蛋白,并与整合素相互作用促进成骨细胞的黏附、增殖和分化[57]。采用微弧氧化技术在钛板上制备含硅的微孔/微球形貌涂层,明显提高了材料表面粗糙度,增强了骨髓间充质干细胞的早期黏附、增殖和成骨分化[58]。涂层中硅掺杂的羟基磷灰石通过上调MAPK和Wnt信号通路促进成骨细胞的增殖和分化,在体内外均被证实能促进成骨细胞的增殖[57]。有学者将硅与具有抗菌作用的Cu一同添加到氧化涂层中,可以在感染条件下维持表面成骨细胞正常的生物学行为,同时该学者还创造性地通过微弧氧化技术将可以增强骨形态发生蛋白2表达并可以促进骨质血管生成的药物(辛伐他汀)加入到氧化层中,从而提高骨质疏松状态下植入物的骨结合能力[39]。 在镁AZ91D合金表面用微弧氧化技术添加的磷酸钙/壳聚糖涂层,通过激活Wnt/β-catenin通路促进大鼠骨髓间充质干细胞的成骨分化、减缓金属基体的降解速率,最终为骨愈合提供了更多时间[59]。通过在Ca3(PO4)2电解液中加入乙二胺四乙酸溶液制备的Ca-P涂层,提高了钙离子和磷酸根的浓度且形成了更厚但微孔更少的氧化层,加强了合金的抗腐蚀性并提高了生物相容性[60]。用微弧氧化技术在纯钽表面制备含钙和磷的多孔Ta2O5层,模拟了骨组织形态和化学成分(Ca/P比值接近1.67),在该涂层表面的小鼠成骨细胞比在纯钽表面的细胞表现出更好的黏附、生长和分化[61-62]。 2.2.2 促纤维生成物质 早期胶原沉积和纤维生成是矿化成骨的重要前提,同时成纤维细胞活性检测也往往用于判断植入物的生物相容性和毒性[63-64]。与纯TiO2涂层相比,采用微弧氧化制备的含Fe3O4纳米颗粒的超顺磁性TiO2涂层,随着Fe3O4含量的增加,成纤维细胞的增殖和细胞外胶原蛋白分泌都有所提高[65]。联合应用微弧氧化、水热处理和退火处理,制备外层为Fe2O3-FeOOH纳米颗粒/内层为Zn掺杂的微孔TiO2的双层多功能涂层,该结构能上调成纤维相关基因(转化生长因子β1、α-平滑肌肌动蛋白和Ⅰ型胶原)的表达,并对成纤维细胞的黏附和增殖有显著的促进作用[66]。联合应用微弧氧化和水热法在钛表面制备以β-FeOOH为外层/Fe-TiO2为内层的复合涂层,体外实验表明该涂层可上调成纤维相关基因(α-平滑肌肌动蛋白、结缔组织生长因子和Ⅰ型胶原)的表达,并且组织镜检发现细胞外胶原蛋白的分泌增加[67]。 2.2.3 促血管生成物质 充足的血供是成骨活动的重要保障,通过微弧氧化技术在钛表面制备出Sr掺杂的纳米孔隙结构,释放的Sr离子可诱导Erk1/2和Akt信号通路的磷酸化,上调犬类骨髓间充质干细胞中低氧诱导因子的表达及维持血管内皮生长因子和血小板衍生生长因子BB蛋白的分泌[68]。有学者用微弧氧化技术在Mg-Ca基底金属的表面固定壳聚糖与矿化胶原(纳米羟基磷灰石/胶原),这种涂层可以提高人脐静脉内皮细胞的迁移能力并增加血管内皮生长因子的表达,并且涂层释放的镁离子可以通过上调人脐静脉内皮细胞中Mg转运蛋白亚型1的表达,进而激活低氧诱导因子来诱导血管生成[69]。 含Cu(0.5 g/L)微弧氧化涂层释放的Cu离子可以使人脐静脉内皮细胞具有良好的迁移能力,并显著提高血管内皮生长因子的产生,促进血管生成[44]。微弧氧化钴(Co)涂层释放的Co离子可以增强间充质干细胞低氧诱导因子和血管内皮生长因子基因的表达和相关蛋白合成,并通过稳定低氧诱导因子来模拟缺氧条件,进而激活血管内皮生长因子基因的表达[70]。如上,Co和Cu均具有促血管新生的能力。学者发现高浓度Cu离子(10 μmol/L)在早期有成血管作用,而低浓度Cu离子(0.1,1 μmol/L)在后期(7 d后)能增强血管生成。高浓度Co离子(25 μmol/L)全程可促进血管生成基因的表达,并且高浓度和低浓度的Co离子(Cu:10 μmol/L,Co:25 μmol/L以及Cu:5 μmol/L,Co:12.5 μmol/L)在一定范围内均可促进管状结构的形成,但联合应用无细胞毒性浓度的Co和Cu离子时,研究者发现血管生成能力无明显变化但细胞毒性反而增加,故不推荐Cu、Co联合应用[71]。 2.3 借助微弧氧化施展新技术 微弧氧化技术与新技术的结合应用弥补了单纯一种技术的缺陷,不仅可以改变涂层的表面形貌,也可改善涂层的生物活性。 2.3.1 微弧氧化与新技术结合改变材料表面形貌 微弧氧化辅助的溶胶-凝胶法可以在镁合金表面制备偏磷酸钙涂层,该涂层不仅能封闭微弧氧化处理后表面的微孔达到提高耐蚀性的结果,还能延缓氢元素对细胞连接和伸展的抑制作用。由于该涂层化学性质与骨组织相似,还可促进人骨髓间充质干细胞在其上的黏附与伸展,最终促进成骨[72]。上述溶胶-凝胶处理虽然可以提高植入体植入成功率并提高耐腐蚀性,但会消除微弧氧化涂层过程中形成的孔隙,影响细胞附着,故有学者先溶胶-凝胶处理、再进行微弧氧化处理来避免该不良结果[73]。 采用微波辅助水热法可在微弧氧化处理的钛表面涂层制备纳米级锐钛矿,该过程调整了微弧氧化表面粗糙度为纳米级,微孔结构增强其对蛋白质的吸附能力(尤其是对于带正电荷蛋白质);在涂层内部的微弧氧化层含有较多的钙、磷离子,可促进细胞早期黏附和伸展,最终促进小鼠骨髓间充质细胞的早期分化[4,74]。 结合微弧氧化与大颗粒喷砂技术可以在钛表面形成火山口状微孔的TiO2膜,获得了更适宜的表面粗糙度,提高了钛的亲水性和表面能(有利于成骨),同时促进种植体周围组织的愈合、骨结合、周围骨质骨小梁微结构的形成,为种植体周围的分子、细胞和组织活性创造了良好的生物活性环境[75]。 结合应用微弧氧化与电喷雾法(利用液体在高电压下充电产生液滴的电场力进行液体雾化)可以在AZ91镁合金表面通过电喷雾制备微弧氧化-聚癸二酸甘油酯双涂层,这种涂层能显著改变微弧氧化层的粗糙度、润湿性、结合力和耐腐蚀性,提高材料表面蛋白的黏附,促进人脐静脉内皮细胞增殖,限制Mg离子释放[76]。 2.3.2 微弧氧化与新技术结合改善涂层生物活性 结合应用微弧氧化技术和水热蒸汽处理技术可制备羟基磷灰石钛合金涂层,该涂层有利于羟基磷灰石的沉积并可促进成骨细胞的黏附、铺展和分化:①促进血管内皮生长因子的分泌来促使内皮细胞生长,从而促进血管生长最终提高骨结合能力;②上调成骨相关基因(骨形态发生蛋白2、Ⅰ型胶原等)和血管生成相关基因(血管内皮生长因子、血管性血友病因子)的表达;③上调骨钙素、蛋白激酶C、活化T细胞核因子和Wnt5的表达,并使成骨细胞通过钙依赖的Wnt信号通路进行分化[77-78]。微弧氧化技术与水热处理技术可以在镁合金氧化涂层上固定具有诱导成骨细胞分化的骨形态发生蛋白2,涂层持续释放的骨形态发生蛋白2可以促进小鼠成骨细胞成骨分化和骨组织生长,当骨形态发生蛋白2质量浓度为50 ng/mL时,植入物的降解速度最慢并在周围形成最均匀的新骨质层,该质量浓度是最有利于骨科植入物和种植牙成活的表面处理浓度[79]。 结合应用微弧氧化与浸涂技术可以在金属锆表面制备具有抗菌作用的壳聚糖层,上述处理后的表面无孔无裂纹并具有疏水性,在体外可显著提高生物活性和磷灰石形成能力[80]。 结合应用微弧氧化和超声技术可提高加入氧化层内的Zn离子释放能力,该处理不仅无细胞毒性,并且在一定程度上增加材料的粗糙度,减小接触角提高表面亲水性,最终提高细胞的早期黏附与表面对蛋白的吸附能力及小鼠成骨细胞的成骨活性[81-82]。"

| [1] GUO X, HU Y, YUAN K, et al. Review of the Effect of Surface Coating Modification on Magnesium Alloy Biocompatibility. Materials (Basel). 2022;15(9):3291. [2] RAZAVI M, FATHI M, SAVABI O, et al. Biodegradable Magnesium Bone Implants Coated with a Novel Bioceramic Nanocomposite. Materials (Basel). 2020;13(6): E1315. [3] ZHAN X, LI S, CUI Y, et al. Comparison of the osteoblastic activity of low elastic modulus Ti-24Nb-4Zr-8Sn alloy and pure titanium modified by physical and chemical methods. Mater Sci Eng C Mater Biol Appl. 2020;113:111018. [4] LIN DJ, FUH LJ, CHEN CY, et al. Rapid nano-scale surface modification on micro-arc oxidation coated titanium by microwave-assisted hydrothermal process. Mater Sci Eng C Mater Biol Appl. 2019;95:236-247. [5] KUANG J, BA Z, LI Z, et al. The study on corrosion resistance of superhydrophobic coatings on magnesium. Appl Surf Sci. 2020;501:144137. [6] PONOMAREV VA, ORLOV EA, MALIKOV NA, et al. Ag(Pt) nanoparticles-decorated bioactive yet antibacterial Ca- and P-doped TiO2 coatings produced by plasma electrolytic oxidation and ion implantation. Appl Surf Sci. 2020;516:146068. [7] NI R, JING Z, XIONG C, et al. Effect of micro-arc oxidation surface modification of 3D-printed porous titanium alloys on biological properties. Ann Transl Med. 2022;10(12):710. [8] PAN X, LI Y, ABDULLAH AO, et al. Micro/nano-hierarchical structured TiO2 coating on titanium by micro-arc oxidation enhances osteoblast adhesion and differentiation. R Soc Open Sci. 2019;6(4):182031. [9] ZHOU W, HUANG O, GAN Y, et al. Effect of titanium implants with coatings of different pore sizes on adhesion and osteogenic differentiation of BMSCs. Artif Cells Nanomed Biotechnol. 2019;47(1):290-299. [10] ZHANG Y, CHEN SE, SHAO J, et al. Combinatorial Surface Roughness Effects on Osteoclastogenesis and Osteogenesis. ACS Appl Mater Interfaces. 2018;10(43): 36652-36663. [11] SHEN X, FANG K, RU YIE KH, et al. High proportion strontium-doped micro-arc oxidation coatings enhance early osseointegration of titanium in osteoporosis by anti-oxidative stress pathway. Bioact Mater. 2022;10:405-419. [12] ZHANG J, SHI H, ZHANG N, et al. Interleukin-4-loaded hydrogel scaffold regulates macrophages polarization to promote bone mesenchymal stem cells osteogenic differentiation via TGF-β1/Smad pathway for repair of bone defect. Cell Prolif. 2020;53(10):e12907. [13] ZHAO Q, SHI M, YIN C, et al. Dual-Wavelength Photosensitive Nano-in-Micro Scaffold Regulates Innate and Adaptive Immune Responses for Osteogenesis. Nanomicro Lett. 2020;13(1):28. [14] MUÑOZ J, AKHAVAN NS, MULLINS AP, et al. Macrophage Polarization and Osteoporosis: A Review. Nutrients. 2020;12(10):2999. [15] ZHAO F, LEI B, LI X, et al. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials. 2018;178:36-47. [16] SOUZA PPC, LERNER UH. The role of cytokines in inflammatory bone loss. Immunol Invest. 2013;42(7):555-622. [17] LI X, HUANG Q, HU X, et al. Evaluating the osteoimmunomodulatory properties of micro-arc oxidized titanium surface at two different biological stages using an optimized in vitro cell culture strategy. Mater Sci Eng C Mater Biol Appl. 2020;110: 110722. [18] ZONG J, HE Q, LIU Y, et al. Advances in the development of biodegradable coronary stents: A translational perspective. Mater Today Bio. 2022;16:100368. [19] ECHEVERRY-RENDON M, ECHEVERRIA F, HARMSEN MC. Interaction of different cell types with magnesium modified by plasma electrolytic oxidation. Colloids Surf B Biointerfaces. 2020;193:111153. [20] PENG F, WANG D, TIAN Y, et al. Sealing the Pores of PEO Coating with Mg-Al Layered Double Hydroxide: Enhanced Corrosion Resistance, Cytocompatibility and Drug Delivery Ability. Sci Rep. 2017;7(1):8167. [21] BONFANTE EA, JIMBO R, WITEK L, et al. Biomaterial and biomechanical considerations to prevent risks in implant therapy. Periodontol 2000. 2019;81(1): 139-151. [22] BRIZUELA A, HERRERO-CLIMENT M, RIOS-CARRASCO E, et al. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials (Basel). 2019;12(6):980. [23] CHEN M, HU Y, HOU Y, et al. Osteogenesis regulation of mesenchymal stem cells via autophagy induced by silica-titanium composite surfaces with different mechanical moduli. J Mater Chem B. 2020;8(40):9314-9324. [24] PAVEL M, RENNA M, PARK SJ, et al. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat Commun. 2018;9(1):2961. [25] TOTARO A, ZHUANG Q, PANCIERA T, et al. Cell phenotypic plasticity requires autophagic flux driven by YAP/TAZ mechanotransduction. Proc Natl Acad Sci U S A. 2019;116(36):17848-17857. [26] LI L, YANG S, XU L, et al. Nanotopography on titanium promotes osteogenesis via autophagy-mediated signaling between YAP and β-catenin. Acta Biomater. 2019;96:674-685. [27] KIANI F, WEN C, LI Y. Prospects and strategies for magnesium alloys as biodegradable implants from crystalline to bulk metallic glasses and composites-A review. Acta biomater. 2020;103:1-23. [28] DOU J, YU H, CHEN C, et al. Preparation and microstructure of MAO/CS composite coatings on Mg alloy. Mater Lett. 2020;271:127729. [29] YANG S, SUN R, CHEN K. Self-healing performance and corrosion resistance of phytic acid/cerium composite coating on microarc-oxidized magnesium alloy. Chem Eng J. 2022;428:131198. [30] WANG Z, YE F, CHEN L, et al. Preparation and Degradation Characteristics of MAO/APS Composite Bio-Coating in Simulated Body Fluid. Coatings. 2021;11(6): 667. [31] FU J, LI M, LIU G, et al. Robust ceramic based self-lubricating coating on Al–Si alloys prepared via PEO and spin-coating methods. Wear. 2020;458-459:203405. [32] TORRENTO JE, GRANDINI CR, SOUSA TSP, et al. Bulk and surface design of MAO-treated Ti-15Zr-15Mo-Ag alloys for potential use as biofunctional implants. Mater Lett. 2020;269:127661. [33] CAI SW, ZONG Y, HUA TS, et al. Study on the inhibition of hydrogen embrittlement of 7050 aluminum alloy in humid air by MAO coating. Anti-Corros Methods Mater. 2020;67(4):387-394. [34] LI CY, FAN XL, ZENG RC, et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31-Influence of EDTA. J Mater Sci Technol. 2019;35(6):1088-1098. [35] CHEN J, ZHANG Y, IBRAHIM M, et al. In vitro degradation and antibacterial property of a copper-containing micro-arc oxidation coating on Mg-2Zn-1Gd-0.5Zr alloy. Colloids Surf B Biointerfaces. 2019;179:77-86. [36] YANG W, GAO Y, GUO P, et al. Adhesion, biological corrosion resistance and biotribological properties of carbon films deposited on MAO coated Ti substrates. J Mech Behav Biomed Mater. 2020;101:103448. [37] PENG F, LI H, WANG D, et al. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg-Al-Layered Double Hydroxide. ACS Appl Mater Interfaces. 2016;8(51):35033-35044. [38] LI XJ, ZHANG M, WEN S, et al. Microstructure and wear resistance of micro-arc oxidation ceramic coatings prepared on 2A50 aluminum alloys. Surf Coat Technol. 2020;394:125853. [39] ZHAO B, LI X, XU H, et al. Influence of Simvastatin-Strontium-Hydroxyapatite Coated Implant Formed by Micro-Arc Oxidation and Immersion Method on Osteointegration in Osteoporotic Rabbits. Int J Nanomedicine. 2020;15:1797-1807. [40] COSTA AI, GEMINI-PIPERNI S, ALVES AC, et al. TiO2 bioactive implant surfaces doped with specific amount of Sr modulate mineralization. Mater Sci Eng C Mater Biol Appl. 2021;120:111735. [41] LI Y, WANG W, YU F, et al. Characterization and cytocompatibility of hierarchical porous TiO2 coatings incorporated with calcium and strontium by one-step micro-arc oxidation. Mater Sci Eng C Mater Biol Appl. 2020;109:110610. [42] ZHANG YY, ZHU Y, LU DZ, et al. Evaluation of osteogenic and antibacterial properties of strontium/silver-containing porous TiO2 coatings prepared by micro-arc oxidation. J Biomed Mater Res B Appl Biomater. 2021;109(4):505-516. [43] ZHOU J, WANG X, ZHAO L. Antibacterial, angiogenic, and osteogenic activities of Ca, P, Co, F, and Sr compound doped titania coatings with different Sr content. Sci Rep. 2019;9(1):14203. [44] LIANG DY, LIANG PC, YI QQ, et al. Copper coating formed by micro-arc oxidation on pure Mg improved antibacterial activity, osteogenesis, and angiogenesis in vivo and in vitro. Biomed Microdevices. 2021;23(3):39. [45] KANG B, LAN D, YAO C, et al. Evaluation of antibacterial property and biocompatibility of Cu doped TiO2 coated implant prepared by micro-arc oxidation. Front Bioeng Biotechnol. 2022;10:941109. [46] SONG J, JIN P, LI M, et al. Antibacterial properties and biocompatibility in vivo and vitro of composite coating of pure magnesium ultrasonic micro-arc oxidation phytic acid copper loaded. J Mater Sci Mater Med. 2019;30(5):49. [47] ZHAO Q, YI L, HU A, et al. Antibacterial and osteogenic activity of a multifunctional microporous coating codoped with Mg, Cu and F on titanium. J Mater Chem B. 2019;7(14):2284-2299. [48] SUN H, YANG Y, YU L, et al. Inhibition of Inflammatory Response and Promotion of Osteogenic Activity of Zinc-Doped Micro-Arc Titanium Oxide Coatings. ACS Omega. 2022;7(17):14920-14932. [49] ZHANG R, XU N, LIU X, et al. Micro/nanostructured TiO2/ZnO coating enhances osteogenic activity of SaOS-2 cells. Artif Cells Nanomed Biotechnol. 2019;47(1): 2838-2845. [50] LI X, WANG M, ZHANG W, et al. A Magnesium-Incorporated Nanoporous Titanium Coating for Rapid Osseointegration. Int J Nanomedicine. 2020;15:6593-6603. [51] ZHANG R, ZHONG S, ZENG L, et al. Novel Mg-Incorporated Micro-Arc Oxidation Coatings for Orthopedic Implants Application. Materials (Basel). 2021;14(19):5710. [52] WANG F, WANG X, XIE E, et al. Simultaneous incorporation of gallium oxide and tantalum microparticles into micro-arc oxidation coating of titanium possessing antibacterial effect and stimulating cellular response. Biomater Adv. 2022;135: 212736. [53] ZHANG X, LV Y, FU S, et al. Synthesis, microstructure, anti-corrosion property and biological performances of Mn-incorporated Ca-P/TiO2 composite coating fabricated via micro-arc oxidation. Mater Sci Eng C Mater Biol Appl. 2020;117: 111321. [54] ZHAO QM, SUN YY, WU CS, et al. Enhanced osteogenic activity and antibacterial ability of manganese-titanium dioxide microporous coating on titanium surfaces. Nanotoxicology. 2020;14(3):289-309. [55] LIU W, CHEN D, JIANG G, Et al. A lithium-containing nanoporous coating on entangled titanium scaffold can enhance osseointegration through Wnt/β-catenin pathway. Nanomedicine. 2018;14(1):153-164. [56] LIU W, LI T, YANG C, et al. Lithium-Incorporated Nanoporous Coating Formed by Micro Arc Oxidation (MAO) on Magnesium Alloy with Improved Corrosion Resistance, Angiogenesis and Osseointegration. J Biomed Nanotechnol. 2019; 15(6):1172-1184. [57] SHEN X, HU W, PING L, et al. Antibacterial and Osteogenic Functionalization of Titanium With Silicon/Copper-Doped High-Energy Shot Peening-Assisted Micro-Arc Oxidation Technique. Front Bioeng Biotechnol. 2020;8:73464. [58] ZHOU W, WANG T, GAN Y, et al. Effect of micropore/microsphere topography and a silicon-incorporating modified titanium plate surface on the adhesion and osteogenic differentiation of BMSCs. Artif Cells Nanomed Biotechnol. 2020;48(1): 230-241. [59] LIU T, LI Y, ZHANG Y, et al. A biodegradable, mechanically tunable micro-arc oxidation AZ91D-based composite implant with calcium phosphate/chitosan coating promotes long-term bone tissue regeneration. Biotechnol J. 2021;16(10): e2000653. [60] JIAN SY, AKTUG SL, HUANG HT, et al. The Potential of Calcium/Phosphate Containing MAO Implanted in Bone Tissue Regeneration and Biological Characteristics. Int J Mol Sci. 2021;22(9):4706. [61] ALMEIDA ALVES CF, FIALHO L, MARQUES SM, et al. MC3T3-E1 cell response to microporous tantalum oxide surfaces enriched with Ca, P and Mg. Mater Sci Eng C Mater Biol Appl. 2021;124:112008. [62] FIALHO L, GRENHO L, FERNANDES MH, et al. Porous tantalum oxide with osteoconductive elements and antibacterial core-shell nanoparticles: A new generation of materials for dental implants. Mater Sci Eng C Mater Biol Appl. 2021;120:111761. [63] LI Y, TAN J, LIU Z, et al. Antibacterial Activity and Cyto-/Tissue-Compatibility of Micro-/Nano-Structured Titanium Decorated with Silver Nanoparticles. J Biomed Nanotechnol. 2018;14(4):675-687. [64] HAN X, ZHANG G, CHAI M, et al. Light-assisted therapy for biofilm infected micro-arc oxidation TiO2 coating on bone implants. Biomed Mater. 2021;16(2):025018. [65] LI K, LIU S, XUE Y, et al. A superparamagnetic Fe3O4-TiO2 composite coating on titanium by micro-arc oxidation for percutaneous implants. J Mater Chem B. 2019;7(34):5265-5276. [66] XUE Y, CHEN J, DING T, et al. Building biointegration of Fe2O3-FeOOH coated titanium implant by regulating NIR irradiation in an infected model. Bioact Mater. 2022;8:1-11. [67] LI K, XUE Y, ZHANG L, et al. β-FeOOH/Fe-TiO2 heterojunctions on Ti for bacteria inactivation under light irradiation and biosealing. Biomater Sci. 2020;8(21): 6004-6016. [68] ZHANG W, CAO H, ZHANG X, et al. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale. 2016;8(9):5291-5301. [69] ZHANG Z, SUN X, YANG J, et al. In vitro evaluation of freeze-drying chitosan-mineralized collagen/Mg-Ca alloy composites for osteogenesis. J Biomater Appl. 2022;36(8):1359-1377. [70] ZHOU J, ZHAO L. Hypoxia-mimicking Co doped TiO2 microporous coating on titanium with enhanced angiogenic and osteogenic activities. Acta Biomater. 2016;43:358-368. [71] BOSCH-RUÉ E, DÍEZ-TERCERO L, RODRÍGUEZ-GONZÁLEZ R, et al. Assessing the potential role of copper and cobalt in stimulating angiogenesis for tissue regeneration. PLoS One. 2021;16(10):e0259125. [72] LIU Y, CHENG X, WANG X, et al. Micro-arc oxidation-assisted sol-gel preparation of calcium metaphosphate coatings on magnesium alloys for bone repair. Mater Sci Eng C Mater Biol Appl. 2021;131:112491. [73] LI R, WEI Y, GU L, et al. Sol-gel-assisted micro-arc oxidation synthesis and characterization of a hierarchically rough structured Ta-Sr coating for biomaterials. RSC Adv. 2020;10(34):20020-20027. [74] LIN DJ, FUH LJ, CHEN WC. Nano-morphology, crystallinity and surface potential of anatase on micro-arc oxidized titanium affect its protein adsorption, cell proliferation and cell differentiation. Mater Sci Eng C Mater Biol Appl. 2020;107: 110204. [75] HE W, YIN X, XIE L, et al. Enhancing osseointegration of titanium implants through large-grit sandblasting combined with micro-arc oxidation surface modification. J Mater Sci Mater Med. 2019;30(6):73. [76] GHAFARZADEH M, KHARAZIHA M, ATAPOUR M. Bilayer micro-arc oxidation-poly (glycerol sebacate) coating on AZ91 for improved corrosion resistance and biological activity. Prog Org Coat. 2021;161:106495. [77] WANG X, MEI L, JIANG X, et al. Hydroxyapatite-Coated Titanium by Micro-Arc Oxidation and Steam-Hydrothermal Treatment Promotes Osseointegration. Front Bioeng Biotechnol. 2021;9:625877. [78] XU L, LI J, XU X, et al. A Novel Cytocompatibility Strengthening Strategy of Ultrafine-Grained Pure Titanium. ACS Appl Mater Interfaces. 2019;11(51):47680-47694. [79] KIM SY, KIM YK, KIM KS, et al. Enhancement of bone formation on LBL-coated Mg alloy depending on the different concentration of BMP-2. Colloids Surf B Biointerfaces. 2019;173:437-446. [80] AKTUG SL, DURDU S, KALKAN S, et al. In vitro biological and antimicrobial properties of chitosan-based bioceramic coatings on zirconium. Sci Rep. 2021; 11(1):15104. [81] HU J, LI H, WANG X, et al. Effect of ultrasonic micro-arc oxidation on the antibacterial properties and cell biocompatibility of Ti-Cu alloy for biomedical application. Mater Sci Eng C Mater Biol Appl. 2020;115:110921. [82] LV Y, SUN S, ZHANG X, et al. Construction of multi-layered Zn-modified TiO2 coating by ultrasound-auxiliary micro-arc oxidation: Microstructure and biological property. Mater Sci Eng C Mater Biol Appl. 2021;131:112487 |
| [1] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [2] | Li Jiaqi, Huang Yuanli, Li Yan, Wang Chunren, Han Qianqian. Mechanism and influencing factors in molecular weight degradation of non-cross-linked hyaluronic acid [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 747-752. |
| [3] | Wang Jiani, Chen Junyu. Angiogenesis mechanism of metal ions and their application in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 804-812. |
| [4] | Zhang Ya, Mu Qiuju, Wang Zilin, Liu Hongjie, Zhu Lili. Hydrogel loaded with platelet-rich plasma promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 690-696. |
| [5] | Yang Yuqing, Chen Zhiyu. Role and application of early transient presence of M1 macrophages in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 594-601. |
| [6] | Li Chengming, Xue Dongling, Yang Xinyu, Xiao Chi, Cui Daping. Mechanism of Chinese medicine for promoting blood circulation and removing blood stasis combined with platelet-rich plasma to improve steroid-induced necrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 288-294. |
| [7] | Pan Chengzhen, Chen Feng, Lin Zonghan, Mo Jian, Zhang Chi, Wei Yuanxun, Wei Zongbo. Mechanism by which terpenoid herbal monomers prevent osteoporosis by regulating nuclear factor-kappaB signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2234-2241. |
| [8] | Zhai Haoyan, Zhao Yuan, Fan Dengying, Liu Chunyan. The role of reactive oxygen species in periodontitis and periodontal tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2254-2260. |
| [9] | Ma Suilu, He Zhijun, Liu Tao, Li Yan, He Yuanxu, He Bo, Wang Weiwei, Wei Xiaotao. Traditional Chinese medicine monomer in the prevention and treatment of flap necrosis by regulating “autophagy” [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 153-158. |
| [10] | Xu Yan, Li Ping, Lai Chunhua, Zhu Peijun, Yang Shuo, Xu Shulan. Piezoelectric materials for vascularized bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1126-1132. |
| [11] | Xu Zhengyi, Wan Qianbing, Chen Junyu. Natural small molecular compounds in the treatment of bone-related diseases by regulating type H blood vessels and its application in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5546-5553. |
| [12] | Chen Bohao, He Qi, Yang Junzheng, Pan Zhaofeng, Xiao Jiacong, Li Miao, Li Shaocong, Zeng Jiaxu, Wang Haibin, Zheng Jia, Zhang Meng. Significance of Piezo1 protein in the pathogenesis of osteonecrosis of femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(27): 4414-4420. |
| [13] | Wang Lijuan, Liu Guobin, Han Qiuqin, Zhao Zhihong, He Jinjing, Li Wenhui. Protective effect of ethanol extract of Zizhu on vascular endothelial cell injury induced by high glucose and high lipids [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(26): 4132-4138. |
| [14] | Wang Wenbo, Xu Jingzhi, Wu Liang, Xi Kun, Xin Tianwen, Tang Jincheng, Gu Yong, Chen Liang. In vitro experiment of composite nanofibrous periosteum to promote vascularization and osteogenic mineralization [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 4028-4037. |
| [15] | He Wenfeng, Xue Cheng, Zheng Jiankang, Shuai Zhuang, Yue Rongchuan. Effects of icariin combined with injectable chitosan/collagen composite hydrogel on angiogenesis in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3992-3998. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||