Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (34): 5571-5576.doi: 10.12307/2024.598
Mechanisms of long non-coding RNA in osteoarthritis and traditional Chinese medicine intervention
Huang Keqi, Li Jiagen, Chen Shangtong, Rong Xiangbin
- Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China
-
Received:2023-08-04Accepted:2023-12-21Online:2024-12-08Published:2024-03-15 -
Contact:Rong Xiangbin, MD candidate, Associate chief physician, Associate professor, Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
About author:Huang Keqi, Master candidate, Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
Supported by:Doctoral Student Innovation Project of Guangxi Zhuang Autonomous Region in 2023, No. YCBZ2023153 (to RXB [project participant]); Guangxi Traditional Chinese Medicine Development and Promotion of Appropriate Technology Project, No. GZSY21-14 (to RXB [project participant])
CLC Number:
Cite this article
Huang Keqi, Li Jiagen, Chen Shangtong, Rong Xiangbin. Mechanisms of long non-coding RNA in osteoarthritis and traditional Chinese medicine intervention[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5571-5576.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
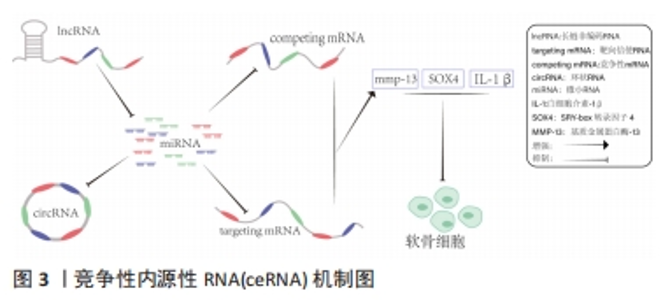
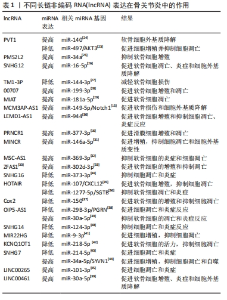
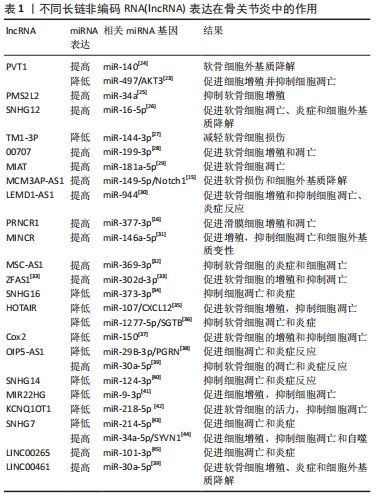
2.1 lncRNA干预骨关节炎的作用机制 lncRNA是长度超过200个核苷酸的ncRNA,占总ncRNA的80%以上,虽然蛋白质编码潜力很小,但在人体内发挥多种生命调控作用[8]。越来越多的研究表明,lncRNA可影响软骨细胞外基质降解、软骨细胞凋亡、滑膜炎和微血管生成,而这些在骨关节炎的发生和发展中起重要作用[9]。CHEN等[10]发现lncRNA HOTAIR参与骨关节炎的发病机制,HOTAIR过表达显著降低白细胞介素1β诱导软骨细胞凋亡和细胞外基质降解。有研究发现,骨关节炎软骨组织中lncRNA FAS-AS1高表达可促进软骨细胞凋亡和外基质的降解。WU等[11]使用基因芯片和生物信息学技术在骨关节炎和非骨关节炎患者膝关节滑膜外泌体中鉴定出196个差异表达的lncRNA,揭示了骨关节炎的发生与lncRNA异常表达密切相关。骨关节炎的主要机制是lncRNA表达增多或者降低,通过ceRNA机制与miRNA结合增多或减少继而引起下游靶基因变化发挥作用,但lncRNA在骨关节炎发展各阶段发挥的不同作用尚待进一步明确。靶向调控lncRNA的新药研发,可能为骨关节炎的治疗提供新的思路。 2.2 ceRNA机制在骨关节炎中的作用 lncRNA常需要通过ceRNA机制与其他RNA竞争发挥作用,所以了解ceRNA对认识lncRNA在骨关节炎中如何发挥作用十分重要。ceRNA机制是一种新兴的基因调控模式,它通过ncRNA(如lncRNA、circRNA和mRNA等)与含有相同miRNA响应元件的竞争性结合来影响靶向基因的表达水平,从而调控细胞生物学过程。在ceRNA机制中,ncRNA与某些靶向基因的miRNA结合,其功能是通过结合到靶向基因的3’UTR区域抑制靶向基因的表达,减少靶向基因的miRNA介导的降解和翻译抑制,从而增加靶向基因的表达水平[12]。同时,ceRNA还可以通过一些转录调控因子来影响其自身及其他基因的表达水平,从而影响细胞的生物学过程。当一个lncRNA作为ceRNA分子竞争miRNA与靶向基因结合时,miRNA就会被占用,无法绑定到其他靶向基因的结合位点上,导致其他RNA分子的表达水平上升。反之,如果miRNA靶向基因结合的RNA分子过多,就会导致其他ceRNA分子被miRNA结合,进而降低其表达水平。通过这种互相竞争的机制,ceRNA分子可以相互调节其在细胞内的表达水平,影响整个基因表达网络的稳态[13],见图3。"
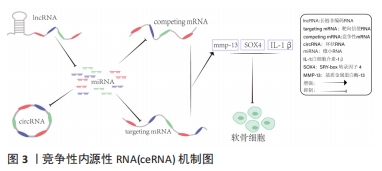
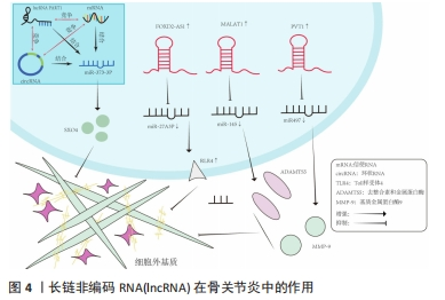
ceRNA机制在许多生物学过程中发挥重要的作用,如细胞增殖、分化、凋亡、炎症反应、癌症发生和发展等。ceRNA机制在骨关节炎中的作用复杂多样,既有促进炎症反应和关节软骨破坏的作用,也有抑制炎症反应、软骨细胞凋亡和促进关节软骨修复的作用[14]。有研究发现,lncRNA MCM3AP-AS1作为miR-149-5p的分子海绵或表观遗传调节因子可以促进跨膜受体蛋白Notch-1的表达;另外,SRY-box 转录因子 4激活的lncRNA MCM3AP-AS1通过靶向miR-149-5p/Notch1轴调节自噬和细胞外基质降解来加重骨关节炎进展[15]。另一研究发现,lncRNA PRNCR1可能通过海绵介导miR-377-3p调控骨关节炎中的细胞增殖、凋亡和白细胞介素1β的表达[16]。LI等[17]通过动物实验表明,circ_0128846作为ceRNA与miR-140-3p和蛋白酪氨酸激酶2(janus kinase 2,JAK2)相互作用,通过上调miR-140-3p和下调骨关节炎细胞中的JAK2来促进胶原蛋白Ⅱ的表达;沉默circ_0128846通过调节miR-140-3p/JAK2轴促进胶原蛋白Ⅱ表达,进而减弱骨关节炎进展。有学者发现Circ_0000423可以直接被miRNA-27b-3p靶向并充当miRNA-27b-3p海绵。Circ_0000423可以通过靶向miRNA-13b-27p表达作为骨关节炎中的ceRNA 来影响Ⅱ型胶原的表达;此外,AAV-shRNA-Circ 0000423关节内注射通过减少关节软骨破坏和侵蚀、关节表面纤维化、骨赘形成、基质金属蛋白酶13 表达和增加关节软骨中胶原蛋白Ⅱ的表达来减缓骨关节炎的进展[18]。基于ceRNA理论的新型人工lncRNA 抑制剂在骨关节炎治疗领域尚处于动物或细胞实验阶段,临床应用还有一定的距离,还需要进一步研究。 2.3 lncRNA通过ceRNA机制干预骨关节炎 在骨关节炎的发病机制中,lncRNA具有调节软骨细胞凋亡增殖、细胞外基质代谢、炎症反应等生物学功能,lncRNA与miRNA之间的相互作用在骨关节炎的发展中起着重要作用。lncRNA可以竞争性结合miRNA并充当ceRNA,减少miRNA与下游基因的组合,增加下游基因的转录和表达[19]。在ceRNA机制中,lncRNA通过与mRNA共享miRNA结合位点,从而调节mRNA的稳定性和翻译效率。lncRNA可以通过相同的miRNA响应元件来调节miRNA的表达。lncRNA的异常表达可对下游基因和通路造成影响,进而影响促进骨关节炎的发生。ZHU等[20]研究发现,lncRNA PART1通过靶向miR-373-3p调节SRY-box转录因子4的表达促进软骨细胞凋亡和细胞外基质降解,进而加重骨关节炎的进展,见图4。WANG等[21]研究发现,lncRNA FOXD2-AS1通过海绵miR-27a-3p上调Toll样受体促进软骨细胞外基质降解,参与骨关节炎进展。LIU等[22]研究发现,lncRNA MALAT1/miR-145轴通过靶向去整合素和金属蛋白酶5(a disintegrin and metalloproteinase with thrombospondin motifs 5,ADAMTS-5)促进白细胞介素1β诱导软骨细胞的细胞外基质降解。关于lncRNA PVT1的研究,XU等[23]发现lncRNA PVT1在骨关节炎患者软骨组织中表达明显升高,其可靶向下调miR-497的表达水平导致聚集聚糖和Ⅱ型胶原蛋白水平降低以及基质金属蛋白酶9水平升高,加剧软骨细胞外基质降解,其中miR-497还受到AKT3的负调控。另一研究发现,骨关节炎患者软骨细胞中miR-140呈低表达和lncRNA PVT1、ADAMTS-5和基质金属蛋白酶13呈高表达,下调lcnRNA PVT1会提高miR-140的表达、降低ADAMTS-5和基质金属蛋白酶13的表达,反之过表达lcnRNA PVT1会出现相反的结果[24]。综合目前研究,lncRNA通过ceRNA机制构成多种复杂的相互作用网络发挥作用,见表1。不同lncRNA通过ceRNA机制与miRNA结合产生不同影响,相同的lncRNA异常表达也可对多种miRNA起到不同的调控作用,引起软骨细胞凋亡、细胞外基质降解、内环境代谢失衡。在骨关节炎发展过程中可见多种lncRNA、miRNA出现异常表达,针对调控lncRNA、miRNA在骨关节炎中的异常表达可能是骨关节炎治疗的新思路,lncRNA在未来可能成为治疗骨关节炎靶点和诊断的生物标志物。但是目前的研究多处于动物实验阶段,缺乏相应的临床试验,另外对于选何种lncRNA作为调控对象、药物的量和安全性尚需要进一步研究。"
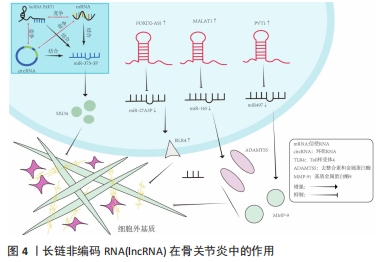
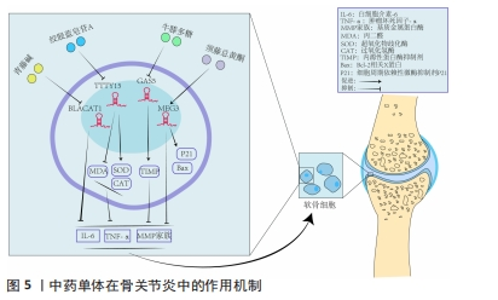
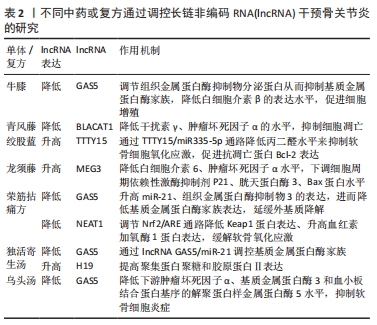
2.4 中药调控lncRNA干预骨关节炎 骨关节炎的临床表现常以痛、关节活动受限为主,在中医中常被称为“骨痹”“骨节病”等。《素问·痹论》中对病因的论述“风寒湿三气杂至,合而为痹也。其风气胜者为行痹,寒气胜者为痛痹,湿气胜者为著痹也”。传统中医药在治疗“骨痹”方面常发挥着巨大的作用。在现代药理学和生物医学领域,中药单体或者复方的有效成分通过增加或抑制lncRNA的表达来影响miRNA和mRNA的表达和功能,降低肿瘤坏死因子α、白细胞介素1β、白细胞介素6等炎性递质的水平,改善骨关节炎的炎症反应[46];同时可提高过氧化物酶、超氧化物歧化酶等氧化还原酶活性,对降低细胞内活性氧水平、减轻氧化应激反应起到重要作用[47],见图5。中医药是如何发挥治疗骨关节炎的作用尚未完全清晰,笔者通过查阅相关文献做出相关综述。不同中药或复方通过调控lncRNA干预骨关节炎的研究,见表2。"
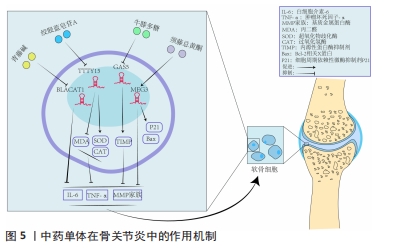

2.4.1 中药单体 (1)青藤碱:是存在于中药青风藤中的一种主要生物碱,具有多种药理活性,如免疫、抗炎、镇痛等作用[48]。陈晨等[49]使用脂多糖诱导软骨细胞建立骨关节炎损伤模型,发现使用生物碱干预后可降低模型中lncRNA BLACAT1的高表达,降低软骨细胞凋亡率及炎症反应,而过表达lncRNA BLACAT1可逆转青藤碱的作用。该实验证明青藤碱可通过抑制lncRNA BLACAT1在骨关节炎中的高表达,减轻细胞凋亡、炎症反应,促进软骨细胞增殖及增强细胞克隆形成能力,从而减轻骨关节炎的进展。 (2)绞股蓝皂苷A:是传统中药绞股蓝的主要有效成分,现代药理研究发现其具有预防衰老、抗血小板聚集、保肝等作用[50]。牛帅帅等[51]通过白细胞介素1β诱导软骨细胞建立骨关节炎模型,发现不同剂量绞股蓝皂苷A干预对软骨细胞的增殖和凋亡有明显影响,绞股蓝皂苷A可下调骨关节炎模型中lncRNA TTTY15的高表达来提升miR-335-5p的表达,降低软骨细胞中肿瘤坏死因子α、白细胞介素6 的水平。丙二醛是一种由于软骨细胞缺血缺氧而导致细胞线粒体发生障碍产生的脂质过氧化产物,而超氧化物歧化酶可清除氧自由基而减轻氧化损伤。牛帅帅等[51]研究还显示造模后的软骨细胞内丙二醛水平升高、超氧化物歧化酶活性降低,而绞股蓝皂苷A干预可明显降低丙二醛水平、增强超氧化物歧化酶活性。当细胞内部的死亡程序启动时,抗凋亡蛋白 Bcl-2 表达水平降低,促凋亡蛋白 Bax 的表达升高,进一步通过 capsase 家族蛋白的活化而执行细胞凋亡。牛帅帅等[51]研究提示绞股蓝皂苷A可抑制Bax表达、促进Bcl-2表达,主要是通过上述3条途径减轻骨关节炎软骨细胞损伤和炎性反应、提高细胞的存活率,并且与药物浓度显著相关,药物浓度与细胞存活率呈正比。刘文斌等[52]发现软骨细胞损伤过程中lncRNA PICSAR表达水平升高,抑制 lncRNA PICSAR表达后软骨细胞活力升高,提示其可能参与损伤过程;使用绞股蓝提取物干预可下调lncRNA PICSAR表达和Cleave-caspase-3 表达水平、降低丙二醛水平、提升超氧化物歧化酶和过氧化氢酶活性,进而减轻软骨细胞损伤和凋亡,表明抑制 lncRNA PICSAR 表达可抑制白细胞介素1β 诱导的软骨细胞凋亡和氧化应激。 综上可知,绞股蓝不仅对心血管疾病有治疗作用,并且绞股蓝及相关提取物可多靶点、多途径对多种lncRNA及其下游通路进行调节,起到治疗骨关节炎的作用,揭示了绞股蓝治疗骨关节炎的机制。 (3)牛膝多糖:牛膝是治疗骨病的传统中药,味辛,性温,主痹症、痿症、膝痛不可屈伸的筋骨疼痛,临床上对骨关节退行性变有良好疗效[52]。牛膝多糖是牛膝中提取的生物活性多糖,具有抗肿瘤、抗衰老、抗凝血及免疫调节等多种药理作用[53]。研究发现lncRNA GAS5在白细胞介素1β处理软骨细胞中的相对表达显著升高,经过牛膝多糖干预后lncRNA GAS5的表达受到显著抑制,并且干预组的基质金属蛋白酶9和基质金属蛋白酶13蛋白表达显著降低、金属蛋白酶组织抑制因子1与金属蛋白酶组织抑制因子3和Ⅱ型胶原蛋白表达显著增加,提示牛膝多糖可以通过抑制lncRNA GAS5的表达来增加细胞外基质合成,维持细胞外基质稳态,缓解骨关节炎期间的关节软骨变性[54]。实验属于动物实验,在临床试验上能是否达到治疗骨关节炎的理想效果仍需进一步实验,但证明lncRNA GAS5可能是治疗骨关节炎的靶点。 (4)龙须藤总黄酮:龙须藤是一种主要针对痹病的特色药,有效成分龙须藤总黄酮具有抗炎、镇痛、抗感染等作用。任安龙等[55]发现白细胞介素1β诱导的软骨细胞中lncRNA MEG3的表达显著降低,白细胞介素6、肿瘤坏死因子α等炎症因子升高,导致软骨细胞死亡,进而促进了骨关节炎进展;龙须藤总黄酮可通过提高lncRNA MEG3表达来抑制caspase-3、Bax、P21蛋白的表达,降低软骨细胞中炎性因子白细胞介素6、肿瘤坏死因子α水平,抑制细胞凋亡,进而达到治疗目的。但该研究仅在细胞水平上进行阐述,关于其具体作用机制仍需进一步研究。 2.4.2 中药复方对LncRNA的干预 中药复方内有多种有效成分,多功效、多靶点、低毒性是治疗骨关节炎的优势。近年来对中药复方调节相关信号通路治疗骨关节炎的机制是研究热点,但中药复方对调节lncRNA机制干预骨关节炎研究较少。作者就相关研究进展综述如下。 (1)荣筋拈痛方:源于《清宫配方集成》,具有 “补肝肾、强筋骨、祛风湿、止痹痛”之功效。现代药理研究表明,荣筋拈痛方有抗炎镇痛、刺激蛋白合成的作用[56]。付长龙等[56]研究发现基质金属蛋白酶3、基质金属蛋白酶9、基质金属蛋白酶13、ADAMTS-5等表达在膝关节炎软骨细胞中显著上升,这些是软骨基质降解、关节软骨退变的关键因子,同时发现软骨细胞模型中lncRNA GAS5的表达明显升高,miR-21及其靶基因基质金属蛋白酶抑制因子3的表达量明显降低;经过荣筋拈痛方干预后,lncRNA GAS5的表达量降低,miR-21、组织抑制金属蛋白酶3的表达量升高,基质金属蛋白酶家族的表达量降低,提示荣筋拈痛方下调lncRNA GAS5并介导下游相关信号通路延缓软骨细胞外基质降解,起到防治膝骨关节炎的作用。付长龙等[57]发现膝骨关节炎模型组软骨组织中lncRNA NEAT1表达显著上升,胫荣筋拈痛方干预后lncRNA NEAT1的水平明显降低,病理检查软骨组织比模型组完整,各层次清晰,提示荣筋拈痛方干预通过下调软骨组织 lncRNA NEAT1表达达到延缓骨关节炎退变速度;荣筋拈痛方的作用机制可能是下调lncRNA NEAT1表达进而调节Nrf2/ARE通路,降低Keap1表达,进而缓解软骨组织氧化应激反应,达到延缓骨关节炎软骨退变的疗效。该研究仍然有不足之处,lncRNA调节Nrf2/ARE通路的作用机制尚未明确,需要更进一步研究。 (2)独活寄生汤:出自孙思邈《备急千金要方》, 临床上常用于骨关节炎治疗,疗效稳定可靠。韩玫等[58]发现独活寄生汤可下调LncRNA GAS5在鼠软骨中的表达,进而上调miR-21基因表达、下调金属蛋白酶家族和ADAMTS表达。大量研究证实,基质金属蛋白酶和ADAMTS表达水平在骨关节炎进展中起到重要作用[59]。独活寄生汤可通过调控 lncRNA GAS5/miR-21表达影响下游调节因子的表达水平,减轻软骨外基质降解,从而发挥治疗作用[60]。聚集蛋白聚糖和胶原蛋白Ⅱ是软骨细胞外基质的重要组成成分,其复杂交错的网状结构可增强软骨的机械强度和抗剪切能力,该两种成分容易被基质金属蛋白酶家族蛋白降解,而独活寄生汤可通过显著上调lncRNA H19和miR-675-3p的表达来提高聚集蛋白聚糖和胶原蛋白Ⅱ表达,逆转基质金属蛋白酶的降解,达到治疗骨关节炎的目的。至于独活寄生汤是如何调控lncRNA H19和miR-675-3p尚需后期进一步深入研究。 (3)乌头汤:出自张仲景的《金匮要略》,是临床治疗骨关节炎的经方之一,全方由制川乌、麻黄、黄芪、芍药和炙甘草组成,方中制川乌为君药,性辛热,主散寒除湿,止关节痹痛。研究发现乌头汤可降低软骨退变中一氧化氮的水平、提高超氧化物歧化酶活性,减轻氧化应激反应,并且通过激活 Keap1/Nrf2信号通路中的关联调节因子发挥抗氧化作用[61]。有学者发现乌头汤可以下调lncRNA GAS5表达水平,降低下游肿瘤坏死因子α、基质金属蛋白酶3和ADAMTS-5水平,进而抑制软骨细胞炎症反应,防止细胞外基质降解,从而延缓关节软骨退变。该研究探讨了乌头汤治疗骨关节炎软骨细胞退变的作用机制,但是未涉及乌头汤与骨关节炎相关证型之间的关系,需要进一步动物实验来对比乌头汤对不同证型小鼠的治疗效果。"

| [1] CHARLIER E, DEROYER C, CIREGIA F, et al. Chondrocyte dedifferentiation and osteoarthritis(OA). Biochem Pharmacol. 2019;165:49-65. [2] GIKARO JM, XIONG H, LIN F. Activity limitation and participation restriction in Osteoarthritis and Rheumatoid arthritis: findings based on the National Health and Nutritional Examination Survey. BMC Musculoskelet Disord. 2022;23(1):647. [3] GOH SL, PERSSON M, STOCKS J, et al. Relative Efficacy of Different Exercises for Pain, Function, Performance and Quality of Life in Knee and Hip Osteoarthritis: Systematic Review and Network Meta-Analysis. Sports Med. 2019;49(5):743-761. [4] 李盛华,周明旺.规范膝骨关节炎的分期分型,倡导膝骨关节炎的中医疗法——《膝骨关节炎中医诊疗指南(2020年版)》解读[J].中医正骨,2021,33(7):1-3. [5] ZHANG F, LAMMI MJ, TAN S, et al. Cell cycle-related lncRNAs and mRNAs in osteoarthritis chondrocytes in a Northwest Chinese Han Population. Medicine (Baltimore). 2020;99(24):e19905. [6] KONG H, SUN ML, ZHANG XA, et al. Crosstalk Among circRNA/lncRNA, miRNA, and mRNA in Osteoarthritis. Front Cell Dev Biol. 2021;9:774370. [7] 刘莉梅,杜小正,方晓丽,等.长链非编码RNA与RA相关性及中医药干预的研究进展[J].中国骨质疏松杂志,2022,28(11):1700-1705. [8] KARAGKOUNI D, KARAVANGELI A, PARASKEVOPOULOU MD, et al. Characterizing miRNA-lncRNA Interplay. Methods Mol Biol. 2021;2372: 243-262. [9] HE CP, JIANG XC, CHEN C, et al. The function of lncRNAs in the pathogenesis of osteoarthritis. Bone Joint Res. 2021;10(2):122-133. [10] CHEN Y, ZHANG L, LI E, et al. Long-chain non-coding RNA HOTAIR promotes the progression of osteoarthritis via sponging miR-20b/PTEN axis. Life Sci. 2020;253:117685. [11] WU X, BIAN B, LIN Z, et al. Identification of exosomal mRNA, lncRNA and circRNA signatures in an osteoarthritis synovial fluid-exosomal study. Exp Cell Res. 2022;410(1):112881. [12] REN S, LIN P, WANG J, et al. Circular RNAs: Promising Molecular Biomarkers of Human Aging-Related Diseases via Functioning as an miRNA Sponge. Mol Ther Methods Clin Dev. 2020;18:215-229. [13] TEHRANI SS, EBRAHIMI R, AL-E-AHMAD A, et al. Competing Endogenous RNAs (CeRNAs): Novel Network in Neurological Disorders. Curr Med Chem. 2021;28(29):5983-6010. [14] LIU Y, GU X, LIU H, et al. New Insight of Circular RNAs in Human Musculoskeletal Diseases. DNA Cell Biol. 2020;39(11):1938-1947. [15] XU F, HU Q F, LI J, et al. SOX4-activated lncRNA MCM3AP-AS1 aggravates osteoarthritis progression by modulating miR-149-5p/Notch1 signaling. Cytokine. 2022;152:155805. [16] WANG G, LI C, ZHANG X, et al. Long non-coding PRNCR1 regulates the proliferation and apoptosis of synoviocytes in osteoarthritis by sponging miR-377-3p. J Orthop Surg Res. 2022;17(1):238. [17] LI H, LIU Z, GUO X, et al. Circ_0128846/miR-140-3p/JAK2 Network in Osteoarthritis Development. Immunol Invest. 2022;51(6):1529-1547. [18] LI X, XIE C, XIAO F, et al. Circular RNA circ_0000423 regulates cartilage ECM synthesis via circ_0000423/miRNA-27b-3p/MMP-13 axis in osteoarthritis. Aging (Albany NY). 2022;14(8):3400-3415. [19] SUN H, PENG G, NING X, et al. Emerging roles of long noncoding RNA in chondrogenesis, osteogenesis, and osteoarthritis. Am J Transl Res. 2019; 11(1):16-30. [20] ZHU YJ, JIANG DM. LncRNA PART1 modulates chondrocyte proliferation, apoptosis, and extracellular matrix degradation in osteoarthritis via regulating miR-373-3p/SOX4 axis. Eur Rev Med Pharmacol Sci. 2019;23(19): 8175-8185. [21] WANG Y, CAO L, WANG Q, et al. LncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging miR-27a-3p in osteoarthritis. Artif Cells Nanomed Biotechnol. 2019;47(1):1241-1247. [22] LIU C, REN S, ZHAO S, et al. LncRNA MALAT1/MiR-145 Adjusts IL-1β-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis. Yonsei Med J. 2019;60(11):1081-1092. [23] XU J, FANG X, QIN L, et al. LncRNA PVT1 regulates biological function of osteoarthritis cells by regulating miR-497/AKT3 axis. Medicine (Baltimore). 2022;101(45):e31725. [24] YAO N, PENG S, WU H, et al. Long noncoding RNA PVT1 promotes chondrocyte extracellular matrix degradation by acting as a sponge for miR-140 in IL-1β-stimulated chondrocytes. J Orthop Surg Res. 2022;17(1):218. [25] YANG F, ZHAO M, SANG Q, et al. Long non-coding RNA PMS2L2 is down-regulated in osteoarthritis and inhibits chondrocyte proliferation by up-regulating miR-34a. J Immunotoxicol. 2022;19(1):74-80. [26] YANG X, CHEN H, ZHENG H, et al. LncRNA SNHG12 Promotes Osteoarthritis Progression Through Targeted Down-Regulation of miR-16-5p. Clin Lab. 2022;68(1):210402. [27] YI Y, YANG N, YANG Z, et al. LncRNA TM1-3P Regulates Proliferation, Apoptosis and Inflammation of Fibroblasts in Osteoarthritis through miR-144-3p/ONECUT2 Axis. Orthop Surg. 2022;14(11):3078-3091. [28] XU Y, DUAN L, LIU S, et al. Long intergenic non-protein coding RNA 00707 regulates chondrocyte apoptosis and proliferation in osteoarthritis by serving as a sponge for microRNA-199-3p. Bioengineered. 2022;13(4): 11137-11145. [29] ZENG S, TU M. The lncRNA MIAT/miR-181a-5p axis regulates osteopontin (OPN)-mediated proliferation and apoptosis of human chondrocytes in osteoarthritis. J Mol Histol. 2022;53(2):285-296. [30] LI H, LIAN K, MAO J, et al. LncRNA LEMD1-AS1 relieves chondrocyte inflammation by targeting miR-944/PGAP1 in osteoarthritis. Cell Cycle. 2022;21(19):2038-2050. [31] LI D, WANG X, YI T, et al. LncRNA MINCR attenuates osteoarthritis progression via sponging miR-146a-5p to promote BMPR2 expression. Cell Cycle. 2022;21(22):2417-2432. [32] TANG Z, GONG Z, SUN X. Long non-coding RNA musculin antisense RNA 1 promotes proliferation and suppresses apoptosis in osteoarthritic chondrocytes via the microRNA-369-3p/Janus kinase-2/ signal transducers and activators of transcription 3 axis. Bioengineered. 2022;13(1):1554-1564. [33] LI J, LIU M, LI X, et al. Long noncoding RNA ZFAS1 suppresses chondrocytes apoptosis via miR-302d-3p/SMAD2 in osteoarthritis. Biosci Biotechnol Biochem. 2021;85(4):842-850. [34] FAN H, DING L, YANG Y. lncRNA SNHG16 promotes the occurrence of osteoarthritis by sponging miR-373-3p. Mol Med Rep. 2021;23(2):117. [35] LU J, WU Z, XIONG Y. Knockdown of long noncoding RNA HOTAIR inhibits osteoarthritis chondrocyte injury by miR-107/CXCL12 axis. J Orthop Surg Res. 2021;16(1):410. [36] WANG B, SUN Y, LIU N, et al. LncRNA HOTAIR modulates chondrocyte apoptosis and inflammation in osteoarthritis via regulating miR-1277-5p/SGTB axis. Wound Repair Regen. 2021;29(3):495-504. [37] JIANG M, XU K, REN H, et al. Role of lincRNA-Cox2 targeting miR-150 in regulating the viability of chondrocytes in osteoarthritis. Exp Ther Med. 2021;22(2):800. [38] ZHI L, ZHAO J, ZHAO H, et al. Downregulation of LncRNA OIP5-AS1 Induced by IL-1β Aggravates Osteoarthritis via Regulating miR-29b-3p/PGRN. Cartilage. 2021;13(2_suppl):1345S-1355S. [39] ZHANG Y, MA L, WANG C, et al. Long noncoding RNA LINC00461 induced osteoarthritis progression by inhibiting miR-30a-5p. Aging (Albany NY). 2020;12(5):4111-4123. [40] WANG B, LI J, TIAN F. Downregulation of lncRNA SNHG14 attenuates osteoarthritis by inhibiting FSTL-1 mediated NLRP3 and TLR4/NF-κB pathway through miR-124-3p. Life Sci. 2021;270:119143. [41] LONG H, LI Q, XIAO Z, et al. LncRNA MIR22HG promotes osteoarthritis progression via regulating miR-9-3p/ADAMTS5 pathway. Bioengineered. 2021;12(1):3148-3158. [42] LIU Y, ZHAO D, WANG X, et al. LncRNA KCNQ1OT1 attenuates osteoarthritic chondrocyte dysfunction via the miR-218-5p/PIK3C2A axis. Cell Tissue Res. 2021;385(1):115-126. [43] XU J, PEI Y, LU J, et al. LncRNA SNHG7 alleviates IL-1β-induced osteoarthritis by inhibiting miR-214-5p-mediated PPARGC1B signaling pathways. Int Immunopharmacol. 2021,90:107150. [44] TIAN F, WANG J, ZHANG Z, et al. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol Res. 2020;53(1):9. [45] ZOU H, LU C, QIU J. Long non-coding RNA LINC00265 promotes proliferation, apoptosis, and inflammation of chondrocytes in osteoarthritis by sponging miR-101-3p[J]. Autoimmunity. 2021;54(8):526-538. [46] LI G, LIU Y, MENG F, et al. Tanshinone IIA promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by up-regulating lncRNA GAS5. Biosci Rep. 2018;38(5): BSR20180626. [47] ZHANG LB, YAN Y, HE J, et al. Epimedii Herba: An ancient Chinese herbal medicine in the prevention and treatment of rheumatoid arthritis. Front Chem. 2022;10:1023779. [48] 刘芝宏,黄玉兰,姜芳,等.青藤碱的合成及构效关系研究进展[J].广州化工,2017,45(6):48-52. [49] 陈晨,郑润泉,李宗玉.青藤碱调控LncRNA BLACAT1对骨关节炎软骨细胞增殖、凋亡的影响[J].中国免疫学杂志,2022,38(17):2069-2073. [50] 张子龙,李雯珊,滕菲,等.绞股蓝总苷提取物及总苷片的质量标准研究[J].中国中药杂志, 2020,45(24):5976-5981. [51] 牛帅帅,孙卓伟,王承群.绞股蓝皂苷A调控lncRNA TTTY15/miR-335-5p通路对骨关节炎软骨细胞损伤的影响及机制[J].河北医药,2022, 44(7):981-985. [52] 刘文斌,李艳兵,周焱涛.绞股蓝提取物通过调控lncRNA PICSAR对IL-1β诱导软骨细胞损伤的保护作用及机制研究[J].疑难病杂志,2021, 20(10):1045-1049. [53] XU XX, ZHANG XH, DIAO Y, et al. Achyranthes bidentate saponins protect rat articular chondrocytes against interleukin-1β-induced inflammation and apoptosis in vitro. Kaohsiung J Med Sci. 2017;33(2):62-68. [54] FU C, QIU Z, HUANG Y, et al. Protective role of Achyranthes bidentata polysaccharides against chondrocyte extracellular matrix degeneration through lncRNA GAS5 in osteoarthritis. Exp Ther Med. 2022;24(2):532. [55] 任安龙,季向荣,方斌.龙须藤总黄酮对膝关节炎软骨细胞增殖、凋亡的影响研究[J].新中医,2020,52(23):12-16. [56] 付长龙,谢新宇,何俊君,等.基于PERK通路探讨荣筋拈痛方对软骨细胞内质网应激反应抑制作用[J]. 福建中医药,2022,53(4):22-24. [57] 付长龙,谢新宇,邱志伟,等.基于lncRNA NEAT1与Nrf2/ARE通路研究荣筋拈痛方延缓膝骨关节炎软骨退变作用机制[J].康复学报,2022, 32(4):332-337. [58] 韩玫,李贞,曹建西.温针灸联合口服独活寄生汤治疗膝骨关节炎寒湿痹阻证[J].中医正骨,2021,33(6):67-69. [59] 李辉,李宁,谢兴文,等.中医药干预基质金属蛋白酶表达治疗膝骨性关节炎研究进展[J].中国骨质疏松杂志,2022,28(1):120-123. [60] 陈俊,郑若曦,叶锦霞,等.独活寄生汤治疗大鼠膝骨关节炎的作用机制研究[J].中医正骨,2021,33(11):6-12. [61] 陈长兴,仲卫红,金灵璐,等.乌头汤抑制膝骨关节炎软骨细胞氧化应激反应的作用研究[J].风湿病与关节炎,2022,11(11):1-4. |
| [1] | Li Yongjie, Fu Shenyu, Xia Yuan, Zhang Dakuan, Liu Hongju. Correlation of knee extensor muscle strength and spatiotemporal gait parameters with peak knee flexion/adduction moment in female patients with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1354-1358. |
| [2] | Du Changling, Shi Hui, Zhang Shoutao, Meng Tao, Liu Dong, Li Jian, Cao Heng, Xu Chuang. Efficacy and safety of different applications of tranexamic acid in high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1409-1413. |
| [3] | Yu Weijie, Liu Aifeng, Chen Jixin, Guo Tianci, Jia Yizhen, Feng Huichuan, Yang Jialin. Advantages and application strategies of machine learning in diagnosis and treatment of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1426-1435. |
| [4] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [5] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [6] | Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300. |
| [7] | Zhang Xiaoyun, Liu Hua, Chai Yuan, Chen Feng, Zeng Hao, Gao Zhengang, Huang Yourong. Effect of Yishen Gushu Formula on bone metabolic markers and clinical efficacyn in patients with osteoporosis of kidney deficiency and blood stasis type [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1155-1160. |
| [8] | Huang Xiarong, Hu Lizhi, Sun Guanghua, Peng Xinke, Liao Ying, Liao Yuan, Liu Jing, Yin Linwei, Zhong Peirui, Peng Ting, Zhou Jun, Qu Mengjian. Effect of electroacupuncture on the expression of P53 and P21 in articular cartilage and subchondral bone of aged rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1174-1179. |
| [9] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [10] | Wang Shanshan, Shu Qing, Tian Jun. Physical factors promote osteogenic differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1083-1090. |
| [11] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [12] | Liu Tao, He Zhijun, Li Jinpeng, Song Yuan, Yao Xingzhang, Chen Wen, Li Yan, Bai Bihui. Role and mechanism of noncoding RNA in diabetic peripheral neuropathy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1124-1129. |
| [13] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [14] | Mei Jingyi, Liu Jiang, Xiao Cong, Liu Peng, Zhou Haohao, Lin Zhanyi. Proliferation and metabolic patterns of smooth muscle cells during construction of tissue-engineered blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1043-1049. |
| [15] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||