Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (31): 4951-4957.doi: 10.12307/2024.181
Previous Articles Next Articles
Notch1-mediated aerobic exercise promotes hippocampal nerve cell proliferation in Alzheimer’s disease mice
Li Huijun1, Li Chuikun1, Wei Cuilan2, Zhang Yeting3
- 1College of Physical Education, Chengdu University, Chengdu 610106, Sichuan Province, China; 2Sports Institute of Chengdu University of Technology, Chengdu 610059, Sichuan Province, China; 3Civil Aviation Flight University of China, Guanghan 618307, Sichuan Province, China
-
Received:2023-04-04Accepted:2023-07-04Online:2024-11-08Published:2024-01-22 -
Contact:Zhang Yeting, PhD, Lecturer, Civil Aviation Flight University of China, Guanghan 618307, Sichuan Province, China -
About author:Li Huijun, Master, Lecturer, College of Physical Education, Chengdu University, Chengdu 610106, Sichuan Province, China
CLC Number:
Cite this article
Li Huijun, Li Chuikun, Wei Cuilan, Zhang Yeting. Notch1-mediated aerobic exercise promotes hippocampal nerve cell proliferation in Alzheimer’s disease mice[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 4951-4957.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
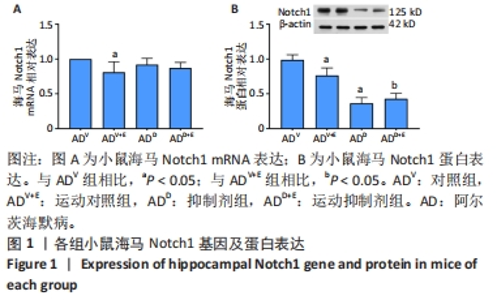
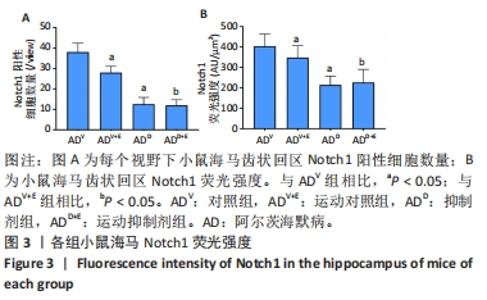
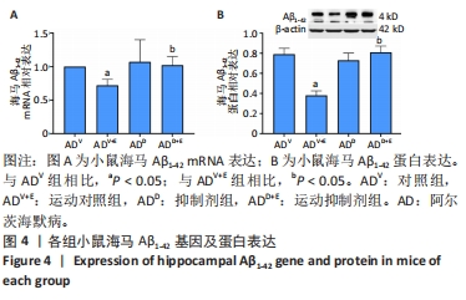
2.1 实验动物数量分析 选用 APP/PS1 双转基因 AD 小鼠共 80 只,分成 4 组,有 2 只 AD 小鼠在实验中死亡 (运动对照组及抑制 剂组各 1 只 ),每组最终各选取 18 只小鼠进行取材。 2.2 小鼠海马Notch1检测结果 图1A为小鼠海马Notch1 mRNA表达:运动对照组显著低于对照组(P=0.003),运动抑制剂组与抑制剂组相比没有显著差异(P=0.331);抑制剂组与对照组相比没有显著差异(P=0.193),运动抑制剂组与运动对照组相比没有显著差异(P=0.304)。图1B为小鼠海马Notch1 蛋白表达:运动对照组显著低于对照组(P < 0.000 5),运动抑制剂组与抑制剂组相比没有显著差异(P=0.198);抑制剂组显著低于对照组(P < 0.000 5),运动抑制剂组显著低于运动对照组(P < 0.000 5)。 "

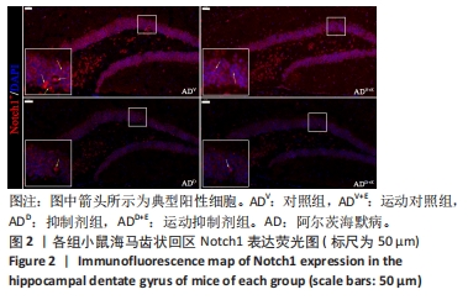
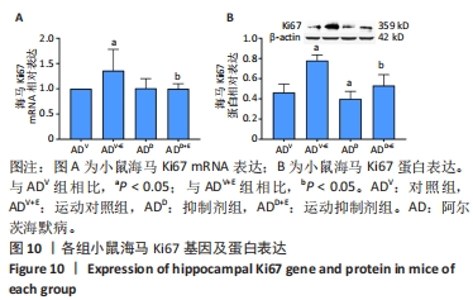
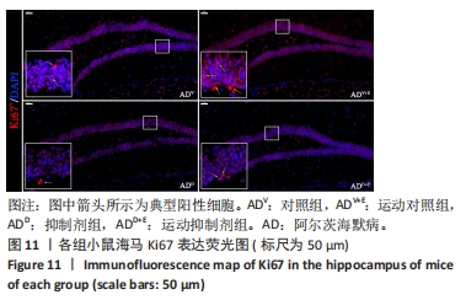
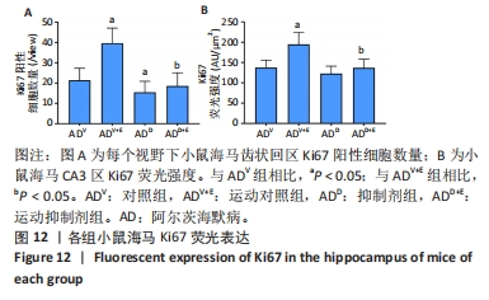
DAPI 标记的细胞核在免疫荧光染色图中呈蓝色,Notch1 免疫阳性产物表达呈红色,见图2。图3A为每个视野下小鼠海马DG区Notch1阳性细胞数量:运动对照组显著低于对照组(P < 0.000 5),运动抑制剂组与抑制剂组相比没有显著差异(P=0.76);抑制剂组显著低于对照组(P < 0.000 5),运动抑制剂组显著低于运动对照组(P < 0.000 5)。图3B为小鼠海马DG区Notch1荧光强度:运动对照组显著低于对照组(P=0.017),运动抑制剂组与抑制剂组相比没有显著差异(P=0.713);抑制剂组显著低于对照组(P < 0.000 5),运动抑制剂组显著低于运动对照组(P=0.002)。"


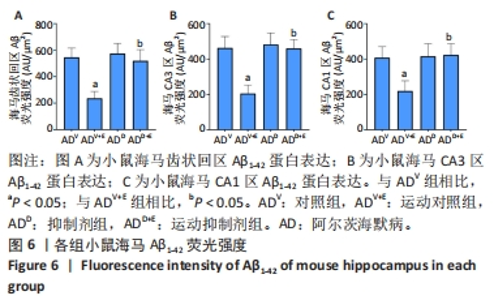
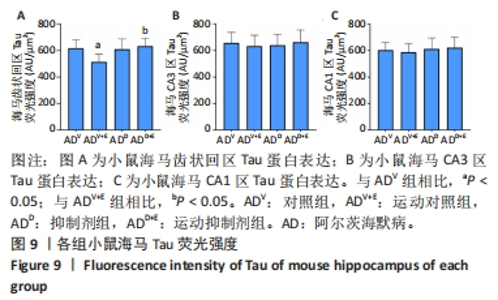
图5中DAPI标记的细胞核呈蓝色,Aβ1-42免疫阳性产物呈红色。小鼠海马DG区的Aβ1-42免疫阳性产物荧光强度:运动对照组显著低于对照组(P < 0.000 5),运动抑制剂组与抑制剂组相比没有显著差异(P=0.196);抑制剂组与对照组相比没有显著差异(P=0.498),运动抑制剂组显著高于运动对照组(P < 0.000 5),见图6A。小鼠海马CA3区的Aβ1-42免疫阳性产物荧光强度:运动对照组显著低于对照组(P < 0.000 5),运动抑制剂组与抑制剂组相比没有显著差异(P=0.519);抑制剂组与对照组相比没有显著差异(P=0.581),运动抑制剂组显著高于运动对照组(P < 0.000 5),见图6B。小鼠海马CA1区的Aβ1-42免疫阳性产物荧光强度:运动对照组显著低于对照组(P < 0.000 5),运动抑制剂组与抑制剂组相比没有显著差异(P=0.828);抑制剂组与对照组相比没有显著差异(P=0.834),运动抑制剂组显著高于运动对照组(P < 0.000 5),见图6C。 "

| [1] SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590. [2] TATULIAN SA. Challenges and hopes for Alzheimer’s disease. Drug Discov Today. 2022;27(4):1027-1043. [3] VAN DYCK CH, SWANSON CJ, AISEN P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9-21. [4] YU W, WANG M, ZHANG Y. Construction of lncRNA-ceRNA networks to reveal the potential role of Lfng/Notch1 signaling pathway in Alzheimer’s disease. Curr Alzheimer Res. 2022. doi: 10.2174/1567205020666221130090103. [5] CHAU DM, CRUMP CJ, VILLA JC, et al. Familial Alzheimer disease presenilin-1 mutations alter the active site conformation of γ-secretase. J Biol Chem. 2012;287(21):17288-17296. [6] WOO HN, PARK JS, GWON AR, et al. Alzheimer’s disease and Notch signaling. Biochem Biophys Res Commun. 2009;390(4):1093-1097. [7] 张业廷,李垂坤,魏翠兰,等. 有氧运动对阿尔茨海默症小鼠成年海马神经发生的影响[J].中国组织工程研究,2024,28(13):2068-2075. [8] GHONEIM FM, KHALAF HA, ELSAMANOUDY AZ, et al. Protective effect of chronic caffeine intake on gene expression of brain derived neurotrophic factor signaling and the immunoreactivity of glial fibrillary acidic protein and Ki-67 in Alzheimer’s disease. Int J Clin Exp Pathol. 2015;8(7):7710-7728. [9] SMITH TW, LIPPA CF. Ki-67 immunoreactivity in Alzheimer’s disease and other neurodegenerative disorders. J Neuropathol Exp Neurol. 1995;54(3):297-303. [10] 张象,张业廷.运动改善阿尔茨海默症模型小鼠病程的剂量效应关系[J].中国组织工程研究,2021,25(17):2761-2766. [11] ZHANG S, WANG P, REN L, et al. Protective effect of melatonin on soluble Aβ1-42-induced memory impairment, astrogliosis, and synaptic dysfunction via the Musashi1/Notch1/Hes1 signaling pathway in the rat hippocampus. Alzheimers Res Ther. 2016;8(1):40. [12] BROWN BM, PEIFFER JJ, MARTINS RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol Psychiatry. 2013;18(8):864-874. [13] YU Q, LI X, WANG J, et al. Effect of exercise training on long-term potentiation and NMDA receptor channels in rats with cerebral infarction. Exp Ther Med. 2013;6(6):1431-1436. [14] ADLARD PA, PERREAU VM, POP V, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25(17):4217-4221. [15] LIU HL, ZHAO G, ZHANG H, et al. Long-term treadmill exercise inhibits the progression of Alzheimer’s disease-like neuropathology in the hippocampus of APP/PS1 transgenic mice. Behav Brain Res. 2013;256:261-272. [16] KANG EB, KWON IS, KOO JH, et al. Treadmill exercise represses neuronal cell death and inflammation during Aβ-induced ER stress by regulating unfolded protein response in aged presenilin 2 mutant mice. Apoptosis. 2013;18(11):1332-1347. [17] ZALOULI V, RAJAVAND H, BAYAT M, et al. Adult hippocampal neurogenesis (AHN) controls central nervous system and promotes peripheral nervous system regeneration via physical exercise. Biomed Pharmacother. 2023;165:115078. [18] PLANCHEZ B, LAGUNAS N, LE GUISQUET AM, et al. Increasing Adult Hippocampal Neurogenesis Promotes Resilience in a Mouse Model of Depression. Cells. 2021;10(5):972. [19] KIM TA, SYTY MD, WU K, et al. Adult hippocampal neurogenesis and its impairment in Alzheimer’s disease. Zool Res. 2022;43(3):481-496. [20] GAMBA P, TESTA G, GARGIULO S, et al. Oxidized cholesterol as the driving force behind the development of Alzheimer’s disease. Front Aging Neurosci. 2015;7:119. [21] BASAK O, GIACHINO C, FIORINI E, et al. Neurogenic subventricular zone stem/progenitor cells are Notch1-dependent in their active but not quiescent state. J Neurosci. 2012;32(16):5654-5666. [22] LI KX, LU M, CUI MX, et al. Astrocyte-neuron communication mediated by the Notch signaling pathway: focusing on glutamate transport and synaptic plasticity. Neural Regen Res. 2023;18(10):2285-2290. [23] TIWARI SK, SETH B, AGARWAL S, et al. Ethosuximide Induces Hippocampal Neurogenesis and Reverses Cognitive Deficits in an Amyloid-β Toxin-induced Alzheimer Rat Model via the Phosphatidylinositol 3-Kinase (PI3K)/Akt/Wnt/β-Catenin Pathway. J Biol Chem. 2015;290(47):28540-28558. [24] ALBERI L, HOEY SE, BRAI E, et al. Notch signaling in the brain: in good and bad times. Ageing Res Rev. 2013;12(3):801-814. [25] CHEN CD, OH SY, HINMAN JD, et al. Visualization of APP dimerization and APP-Notch2 heterodimerization in living cells using bimolecular fluorescence complementation. J Neurochem. 2006;97(1):30-43. [26] SWAMINATHAN B, YOUN SW, NAICHE LA, et al. Endothelial Notch signaling directly regulates the small GTPase RND1 to facilitate Notch suppression of endothelial migration. Sci Rep. 2022;12(1):1655. [27] BRAI E, ALINA RAIO N, ALBERI L. Notch1 hallmarks fibrillary depositions in sporadic Alzheimer’s disease. Acta Neuropathol Commun. 2016; 4(1):64. [28] FAZIO C, RICCIARDIELLO L. Inflammation and Notch signaling: a crosstalk with opposite effects on tumorigenesis. Cell Death Dis. 2016; 7(12):e2515. [29] PAZOS MC, ABRAMOVICH D, BECHIS A, et al. Gamma secretase inhibitor impairs epithelial- to-mesenchymal transition induced by TGF-β in ovarian tumor cell lines. Mol Cell Endocrinol. 2017;440:125-137. [30] AGUAYO-ORTIZ R, GUZMÁN-OCAMPO DC, DOMINGUEZ L. Toward the Characterization of DAPT Interactions with γ-Secretase. ChemMedChem. 2019;14(10):1005-1010. [31] EVIN G, SERNEE MF, MASTERS CL. Inhibition of gamma-secretase as a therapeutic intervention for Alzheimer’s disease: prospects, limitations and strategies. CNS Drugs. 2006;20(5):351-372. [32] HOOK VY, KINDY M, HOOK G. Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J Biol Chem. 2008;283(12):7745-7753. [33] 陈玉晴. γ分泌酶抑制剂DAPT对海马突触传递和活性依赖可塑性的作用及其机制研究[D].上海:复旦大学,2013. [34] 张业廷,魏翠兰,李垂坤,等.Notch1介导有氧运动改善阿尔茨海默病小鼠海马超微结构与空间记忆能力[J].中国康复医学杂志, 2023,38(7):885-897. [35] LANZ TA, HIMES CS, PALLANTE G, et al. The gamma-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester reduces A beta levels in vivo in plasma and cerebrospinal fluid in young (plaque-free) and aged (plaque-bearing) Tg2576 mice. J Pharmacol Exp Ther. 2003;305(3):864-871. [36] 陈天睿,黄雪媚,崔理立,等.治疗阿尔茨海默病的早老素/γ-分泌酶抑制剂和调节剂[J].生命科学,2009,21(4):508-512. |
| [1] | Wang Ji, Zhang Min, Li Wenbo, Yang Zhongya, Zhang Long. Effect of aerobic exercise on glycolipid metabolism, skeletal muscle inflammation and autophagy in type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1200-1205. |
| [2] | Li Longyang, Zhang Songjiang, Zhao Xianmin, Zhou Chunguang, Gao Jianfeng. Electroacupuncture intervention on the proliferation and differentiation of hippocampal neurons and oligodendrocytes in Alzheimer’s disease model mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1029-1035. |
| [3] | Wen Huaneng, Lin Run, Wang Yixiao, Wang Bingshui, Liu Lu, Liu Chuanyao, Cai Canxin, Cui Shaoyang, Xu Mingzhu. Effects of electroacupuncture with “Zhi San Zhen” on Notch signaling pathway and synaptic plasticity in 5xFAD mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5148-5153. |
| [4] | Zhang Jinpu, Wang Junli, Zhang Siqi, Chen Jiahao, Yang Qiushi. Effectiveness of exercise interventions for fibromyalgia syndrome: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5210-5216. |
| [5] | Yang Liyuan, Zhang Yeting, Li Chuikun, Wei Cuilan. Effects of aerobic exercise on the expression of Notch1 and Caspase-3 in the hippocampus of Alzheimer’s disease mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(26): 4113-4120. |
| [6] | Chai Shifan, Li Xinru, Ye Yucai, Sun Junli, Cai Hongyan, Wang Zhaojun. Silent information regulator 1: A potential target of semaglutide in the treatment of Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(20): 3235-3239. |
| [7] | Sun Yuan, Wang Qingbo, Pi Yihua, Lu Chunmin, Xu Chuanyi, Zhang Yan. Effects of early and late aerobic exercise on right heart failure induced by monocrotaline in rats with pulmonary hypertension [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 177-185. |
| [8] | Wang Jingfeng, Wen Dengtai, Wang Shijie, Gao Yinghui. Atg-mediated autophagy, exercise and skeletal muscle aging [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 295-301. |
| [9] | Xiao Yi, Lu Shuo, Ge Lite, Lu Ming. Interaction between autophagy and mesenchymal stem cells in treatment of neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 3111-3116. |
| [10] | Tang Liang, Wang Hexia, Wang Qingbo, Pi Yihua, Zhang Yan. Aerobic exercise modulates mitochondrial quality control system to reverse cardiac pathological remodeling in aging rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2534-2541. |
| [11] | Han Weina, Xu Xiaoqing, Shi Jinning, Li Xinru, Cai Hongyan. Prediction and validation of potential targets for the glucagon-like peptide-1 receptor agonist in the treatment of Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2568-2573. |
| [12] | Wu Yuzhen, Sun Qing, Liu Xia, Zhou Yu, Jin Qiguan. Variation in renal function of type 2 diabetic rats undergoing aerobic exercise [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2145-2151. |
| [13] | Deng Longfei, Zhang Yeting, Fu Yan. Aerobic exercise inhibits neuroinflammation and alleviates cognitive impairment in Alzheimer’s disease model mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2209-2214. |
| [14] | Zhang Yeting, Li Chuikun, Wei Cuilan, Fu Yan, Zhang Feifei. Effects of aerobic exercise on adult hippocampal neurogenesis in Alzheimer’s disease mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2068-2075. |
| [15] | Long Qingxi, Zhang Pingshu, Liu Qing, Ou Ya, Zhang Lili, Yuan Xiaodong. Single-cell RNA sequencing reveals the heterogeneity of astrocytes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 139-146. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||