Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (7): 1135-1141.doi: 10.3969/j.issn.2095-4344.2182
Previous Articles Next Articles
In vitro culture and purification of Schwann cells: a systematic review
Zeng Yanhua1, Hao Yanlei2
- 1College of Clinical Medicine, Jining Medical University, Jining 272000, Shandong Province, China; 2Department of Neurology, Affiliated Hospital of Jining Medical University, Jining 272000, Shandong Province, China
-
Received:2020-04-24Revised:2020-04-28Accepted:2020-05-28Online:2021-03-08Published:2020-12-09 -
Contact:Hao Yanlei, MD, Professor, Chief physician, Department of Neurology, Affiliated Hospital of Jining Medical University, Jining 272000, Shandong Province, China -
About author:Zeng Yanhua, Master candidate, College of Clinical Medicine, Jining Medical University, Jining 272000, Shandong Province, China -
Supported by:the National Natural Science Foundation of China, No. 81771360
CLC Number:
Cite this article
Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141.
share this article

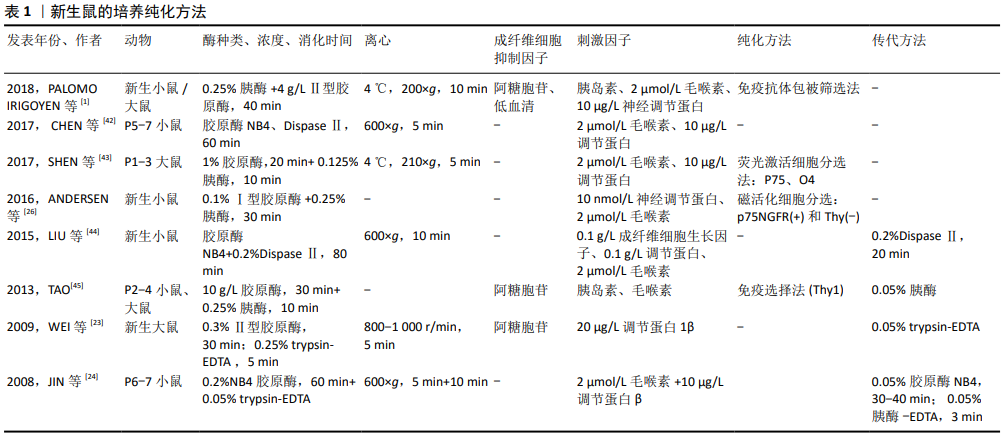
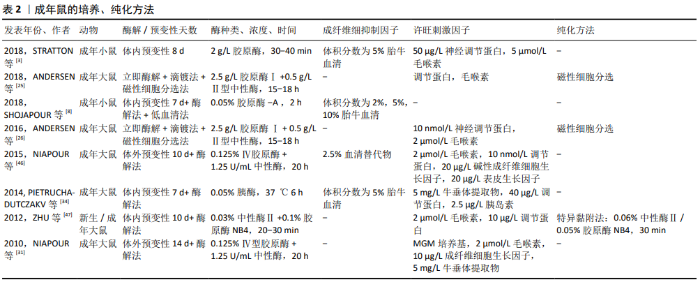
2.1 许旺细胞培养的影响因素 2.1.1 细胞来源 目前许旺细胞培养的宿主来源主要集中在C57BL/6小鼠、大鼠、鸡、犬、兔、猴以及人本身。一般来说,进化越高级,发育越成熟,许旺细胞培养难度越大[14]。 2.1.2 取材部位 许旺细胞主要的取材部位是坐骨神经、腓肠神经、背根神经节及浅表神经。坐骨神经较易获取,但神经外膜完全剥离较困难,体外分离耗时较长而影响细胞活性;背根神经节几乎无神经外膜,获得的许旺细胞的数量、纯度及活性都优于坐骨神经[15]。此外,有研究显示来源于感觉神经的许旺细胞的神经生长因子表达水平要优于运动神 经[16]。有学者用体外冲击波分别处理运动神经来源和感觉神经来源的许旺细胞,发现体外冲击波可诱导运动神经来源的许旺细胞增殖,但感觉神经来源的许旺细胞增殖能力较差,其髓鞘相关因子的表达显著增加[17]。 2.1.3 动物年龄 动物取材年龄有胚胎期、新生期、成年期等。许旺细胞在生长发育的不同阶段及损伤修复过程中表达的标记不同,表明许旺细胞并不是一个功能结构同质体,在不同阶段有不同的增殖能力[18-19]。新生动物神经易分离,且由于其许旺细胞髓鞘还未分化完全,具有较强的增殖能力,因此许旺细胞的分离多取材于胚胎及新生动物。成年动物的正常神经纤维中许旺细胞为终末期细胞,处于静止期,其贴壁及增殖能力很弱。但神经受损变性时许旺细胞开始去分化并大量增殖,产生各种神经生长因子以利于轴突再生,同时巨噬细胞参与许旺细胞有丝分裂因子的产生,进一步加快许旺细胞的增殖[20]。因此成年动物中许旺细胞的培养多应用体内或体外预变性方法,以使许旺细胞进入去分化、增殖状态。 2.1.4 酶种类及消化时间 酶消化法包括单酶和双酶消化法,神经纤维的酶解是影响细胞产量和活性最重要的步骤。对于每一种酶制剂,都需要控制和优化消化解离的时间进程,增加消化时间或提高酶的浓度可以提高细胞的分离数量,但可能会损害其增殖能力。新生动物常用的酶解法是以1979年Brockes方法为基础[10],用胰酶、胶原酶消化新生大鼠的坐骨神经,加入AraC抑制成纤维细胞,抗Thy1抗体及补体去除成纤维细胞后,7 d时许旺细胞纯度可达80%-98%(取决于初始细胞密度),可增殖6代,生存期可达150 d,传代后密度可达99.5%。有研究证明双酶(胰酶联合胶原酶[21];胶原酶联合中性酶)酶解效果优于单用胰酶、胶原酶或中性酶[10,13,22],有学者认为胰酶的消化效果优于胶原酶[23],也有学者认为胶原酶的消化效果优于胰 酶[24],最适合的酶浓度及消化时间尚无明确共识。 2.1.5 包被 层粘连蛋白可促进许旺细胞的活力和生长速 度[24]。有研究显示,与仅涂有多聚赖氨酸的培养皿相比,用多聚赖氨酸和层粘连蛋白顺序包被,黏附性更好,初始群体的存活率也更高[25]。早期许旺细胞可在层粘连蛋白底物上附着和延伸突起,而大多数髓鞘碎片仍然漂浮着,这有利于减少髓鞘碎片的污染[26]。 2.1.6 胎牛血清体积分数 不同体积分数的胎牛血清被认为对培养许旺细胞具有不同程度的影响。NEEDHAM等[27]首次发现无血清培养基(S4 medium)可抑制成纤维细胞过度生长;KOMIYAMA等[28] 证实胎牛血清体积分数为2.5%时,促进许旺细胞增殖及抑制成纤维细胞的效果最好。HEDAYATOUR等[29]对比体积分数为10%,5%,1.25%,0.625%胎牛血清对许旺细胞的影响,发现体积分数10%胎牛血清可得到密度最高的许旺细胞,体积分数2.5%胎牛血清可得到最高纯度的许旺细胞。SHOJAPOUR等[8]对体内预变性的成年小鼠神经酶解后,分别对比体积分数2%,5%,10%胎牛血清对许旺细胞增殖的影响,发现体积分数2%的胎牛血清可获得最高纯度的许旺细胞。以上文献显示,低体积分数的胎牛血清可在一定程度上抑制成纤维细胞增殖,进而促进许旺细胞生长,但高德坤等[30]发现在纯血清中许旺细胞能很快从植块中“爬”出,而成纤维细胞的数量极少,可能的原因是纯血清中含有足量的细胞因子及特殊血清成分,这些成分可增加许旺细胞的贴壁能力,抑制成纤维细胞的长出。 2.1.7 培养基 KOMIYAMA等[28]采用了最初用于培养少突胶质细胞的BS培养基(Bottenstein-Sato medium),并证明该培养基即使在较低的胎牛血清体积分数下也能促进许旺细胞的增殖。NIAPOUR等[31]、Kraus等[32]应用了黑素细胞生长培养基(MGM)以防止成纤维细胞增殖。HAASTERT-TALINI等[33]认为DMEM可诱导成纤维细胞过度生长,而用MGM代替DMEM作为培养基,从预变性的成年鼠坐骨神经中获得90%的许旺细胞。2014,PIETRUCHA-DUTCZAKV等[34]应用了内皮细胞培养基(EBM-2),发现许旺细胞在EBM-2培养基中的生长速度比成纤维细胞快,避免使用抗有丝分裂剂,最终获得94%-97%纯度的许旺细胞。 此外,许旺细胞对培养基的碱化高度敏感,碱性条件下细胞存活率降低,优选在体积分数为8%-9%CO2中(体积分数为10%CO2[19],体积分数为9%CO2[26])孵育细胞以防止pH值变化,操作过程中应避免培养基过度暴露在空气中而碱化,可用含酚红的缓冲液来监控pH值[35]。 2.1.8 初始细胞密度 许旺细胞是自分泌细胞,一旦它们高密度融合,就会自分泌各种神经营养因子如神经生长因子、胰岛素样生长因子、神经刺激素3等,可进一步刺激自身增殖及促进轴突生长[28]。HEDAYATPOUR等[29]证明原代许旺细胞的初始存活率和增殖率取决于培养中的细胞密度。SASAGASAKO等[36]认为1 mL培养基中铺板104-105个细胞数可得到较多许旺细胞。 2.1.9 刺激因子 可溶性生长因子的发现,使许旺细胞的大规模培养成为可能,这些生长因子可维持许旺细胞增殖数代。Heregulin可模拟体内与轴突结合的神经调节素(一种许旺细胞的天然有丝分裂因子),促进许旺细胞的有丝分裂,有实验证明许旺细胞与Hereglin-1β联合移植可促进受损周围神经修复[37]。Forskolin是腺苷酸环化酶的直接激活剂,也是细胞内cAMP的诱导剂,可抑制成纤维细胞的S期进展,减少成纤维细胞的污染并与Heregulin协同促进许旺细胞增殖[38]。胰岛素样生长因子对神经细胞具有营养作用。周围神经损伤后,胰岛素样生长因子在损伤神经中的表达增加,提示其促进轴突再生[39]。胰岛素作为肽激素,亦是一种神经营养因子,对维持中枢和外周神经系统中神经元的稳态有效且关键,胡华麟等[40]发现培养基中加入高糖及胰岛素与单纯高糖环培养基明显有利于许旺细胞的再生,这表明胰岛素在周围神经的再生中具有重要作用。 2.2 许旺细胞的纯化 2.2.1 新生鼠 新生动物来源的许旺细胞增殖能力明显高于类似条件下培养的成年动物的细胞,新生鼠在许多研究中用作许旺细胞培养的动物来源。1991,KOMIYAMA等[41]对比出生3,10,30 d小鼠坐骨神经来源的许旺细胞,发现在体积分数为15%胎牛血清的培养基中,出生3 d小鼠来源的许旺细胞增殖及贴壁能力最强,且小鼠许旺细胞对胸腺嘧啶核苷的摄取是同龄大鼠的3倍。这表明大鼠许旺细胞纯化方法可能不同于小鼠,如抗有丝分裂剂不适用于具有更高增殖特性的小鼠许旺细胞的纯化,因为它会同时损害高度增殖的许旺细胞和成纤维细胞。尽管有研究显示新生小鼠来源的许旺细胞增殖能力更强,但由于其神经细小,剥离困难,去除外膜耗费时间长,且后期的抗有丝分裂法对其活性损害更大,大部分文献中采用新生大鼠作为许旺细胞培养来源。 分离培养方面,新生鼠多采用酶解法,但酶的浓度、酶解时间及离心步骤多样,该综述纳入文献涉及的酶种类及浓度有0.25%胰酶、0.125%胰酶、0.25% trypsin-EDTA、0.05% trypsin-EDTA、 0.1%Ⅰ型胶原酶、0.3%Ⅱ型胶原酶、4 g/L胶原酶、10 g/L胶原酶、0.2%NB4胶原酶、0.2%Ⅱ型中性酶等,酶解时间从5 min到60 min不等。酶解时间过短,组织消化不完全,不利于细胞分散,消化时间过长则会损害细胞增殖活性及质量,根据作者实验室经验,酶解时间需根据显微镜下的观察结果来确定,在酶解至组织分散一定程度时,可用巴氏管轻微吹打。 关于纯化,主要分为抑制成纤维杂质细胞及促进许旺细胞增殖2个方面,该综述纳入文献中多利用抗有丝分裂剂阿糖胞苷抑制成纤维杂质细胞,2018年PALOMO IRIGOYEN等[1]联合利用了阿糖胞苷及低血清法;促进许旺细胞增殖方面,多在培养基中加入刺激因子如胰岛素、毛喉素、调节蛋白、神经调节蛋白等,纯度基本相同。除了在培养基中添加化学物质之外,一些文献利用了特殊仪器或技术进一步纯化,如荧光激活细胞分选法(FACS)是将细胞经免疫荧光染色后放入流式细胞仪中,根据所染荧光团的类型,可将荧光团偶联抗体染色细胞彼此分离;磁活化细胞分选法(MACS)是基于细胞表面抗原能与连接有磁珠的特异性单抗相结合,结合的细胞在外加磁场作用下被吸附,从而使细胞得以分离;免疫选择法(Thy1)是利用成纤维细胞上表达Thy1抗原而许旺细胞不表达的原理,后期加入补体可选择性杀死成纤维细胞。新生鼠各文献的培养、纯化方式见表1。"
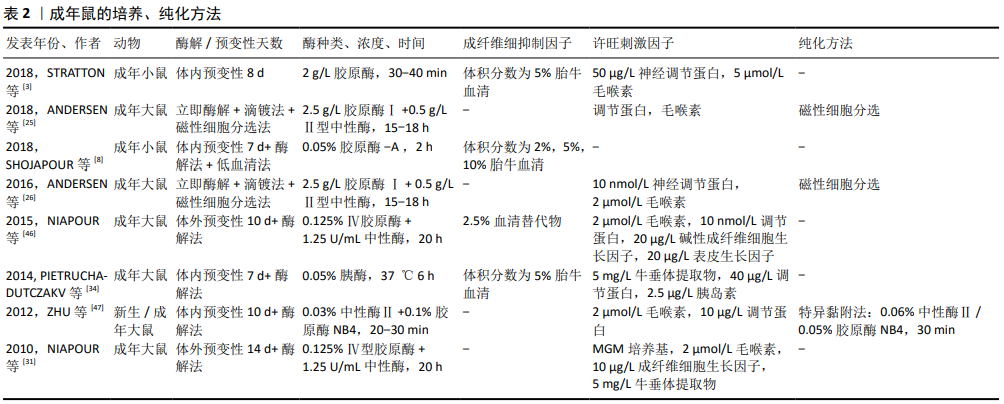
2.2.2 成年鼠 使用成年动物作为许旺细胞来源更具挑战,一是髓鞘分化完全,细胞较难分离;二是存在发育成熟的神经膜及结缔组织,阻碍酶解活性,且富含成纤维细胞;三是细胞处于静止期,酶解出的活性细胞产量低,增殖能力差。因此成年动物神经组织的消化和髓鞘的去除,往往需要更大程度的机械分离、更高的酶浓度、更长的消化时间和更多的髓鞘纯化步骤,而这些会影响细胞的增殖活性[26]。已经证明,这些挑战可通过体内预变性的步骤来克服,神经发生华勒变性,许旺细胞进行去分化、增殖和髓鞘降解,从而在后续培养中获得更高的许旺细胞活性和产量,减少成纤维细胞污染[46]。 近年来,成年鼠的体外培养方法有了很大进展,分离的神经即时酶解后可进行体外预变性,这避免了体内预变性的2次手术步骤的繁杂及不利因素。但预变性延迟了许旺细胞的释放,且长期缺氧可能损害许旺细胞的活性和增殖能力。目前预变性时间并未统一,KOMIYAMA等[28]预变性21 d 获得高纯度许旺细胞。SHOJAPOUR等[8]、NIAPOUR等[46],KRAUS等[32]认为预变性1周时获得的许旺细胞数量最多,活性也较好。TOMKO等[22]通过对比预变性0,2,4,6周获得的许旺细胞数量及活性,认为预变性时间越长,许旺细胞就越活跃,成纤维细胞的增殖能力就越差。 2016年,ANDERSEN等[26]首次联合酶解法及“滴镀法”进行原代细胞铺板,从而避免体内或体外预变性的复杂操作及不利因素,其具体步骤是在显微镜下用镊子将分离出的神经立即缓慢梳理为单纤维,再用胶原酶及中性酶将神经单纤维消化为细胞悬浮液,最后将获得的细胞悬浮液行“滴镀法”铺板,即以一定密度逐滴滴在提前包被的培养皿中。滴镀法可将神经分离成单纤维,促进酶消化完全,以确保高度活性的单个细胞释放,在防止髓鞘碎屑附着的同时,提高了单位表面积的细胞密度,有助于贴壁细胞的存活[25-26]。成年鼠各文献的培养、纯化方式见表2。"
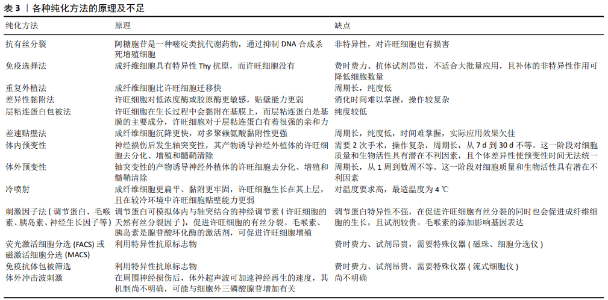
2.2.3 纯化方法 到目前为止,主要有以下几种纯化许旺细胞的方法,包括抗有丝分裂法、免疫选择法、重复外植法、特异黏附法、体内或体外预变性、冷喷射技术、差速贴壁法、层粘连蛋白包被法、低血清法、刺激因子法、荧光激活细胞分选或磁激活细胞分选、免疫抗体包被筛选法、体外冲击波刺激法。 这些方法都可以在一定时间富集不同纯度和产率的许旺细胞,但都存在一定的不足:①抗有丝分裂剂可抑制成纤维细胞增殖,但不能去除那些生长在许旺细胞层下的成纤维细胞,且有报道显示这些抗有丝分裂剂缺乏特异性,会对许旺细胞产生一定的毒性,可阻断神经生长因子转导,影响神经的再生,因此不适合在临床上推广[48];②免疫选择法中,利用Thy抗体与补体介导的细胞溶解可以去除成纤维细胞,但对于大规模的制备来说成本太高,从而限制了其在临床上的大规模应用;③免疫细胞分选法、磁性细胞分选法是制备高纯度许旺细胞的好方法,磁性细胞分选法利用结合许旺细胞表面特有的p75抗原,在外加磁场的吸引下定向移动,从而达到分离提纯许旺细胞的目的,但需要昂贵的抗体和特殊的设备(磁性离心器、免疫磁珠)[49];④免疫抗体包被筛选法是2011年Ben Barres实验室提出用来分离星形胶质细胞的方法,需要约6 h的手动操作时间,该方法得到的细胞可立即使用,纯度很高,但产量较低,也更昂贵(抗体及生长因子),还需要特殊仪器(流式细胞仪)[45];⑤反复植块法、体内或体外预变性、差异性黏附需要相对复杂或耗时的操作,可能会导致许旺细胞丢失,无法在一定的时间达到理想的细胞产量;⑥冷喷射和差速贴壁技术是培养许旺细胞的相对简单的方法,但在短时间内对许旺细胞进行频繁的操作可能会导致许旺细胞的产量较低,特别是在初期操作不熟练时。各种纯化方法的原理及不足见表3。 体外冲击波刺激法最近被发现在周围神经再生过程中有积极作用[50],其最初用于泌尿外科粉碎肾结石,但临床前和临床研究都表明,体外冲击波疗法将是再生医学中的一种有效疗法,如治疗缺血性组织坏死或慢性伤口[51]。HAUSNER等[52]发现在周围神经损伤部位进行自体神经移植后,体外冲击波直接照射损伤部位可显著促进功能恢复。SCHUH等[53]首次证明体外冲击波可显著提高许旺细胞体外培养纯度和增殖能力。经体外冲击波疗法处理的神经来源许旺细胞,培养质量有明显改善,增殖率及纯度较高,再生表型标记明显。WEIHS等[54]阐述了体外冲击波疗法可通过ATP释放偶联的细胞外信号调节激酶(ERK)激活来刺激细胞增殖。HERCHER等[17]证明在自体移植物上使用体外冲击波刺激法处理,可提高感觉神经来源的许旺细胞的再生能力。 2.2.4 许旺细胞的鉴定 目前,鉴定许旺细胞的方法主要为细胞形态学观察和免疫学标记方法。许旺细胞在光镜下体积小,多为双极,倒置相差显微镜下许旺细胞多呈长梭形,也可呈现长三角形或圆形,核为长梭形或椭圆形,胞浆少,细胞边缘发亮,两端突起细长并相互连接或排列成束,容易识别[44]。 免疫学标记是最重要的鉴定方法,利用许旺细胞与成纤维细胞所表达结合的表面抗原不同的原理,S100和P75NTR是最常用的表面标志蛋白。LIU等[44]分析了许旺细胞特异性标志物的表达及其与许旺细胞生长阶段的关系。结果表明,在原代许旺细胞培养早期,S100和P75NTR的阳性表达率分别为(85.84±1.86)%和(93.57±3.40)%,培养3代(8 d)后这2种标志物的阳性率为100%。由于S100在早期培养的许旺细胞中没有完全表达,而且其他多种细胞,如软骨细胞、脂肪细胞也在体内表达S100,因此在培养早期S100不能单独作为鉴定许旺细胞的标记物,而转录因子Sox10在许旺细胞所有阶段中均有表达,且在体内外均有稳定的表达,不易受环境影响,因此它可以作为鉴定许旺细胞的标记物。"

| [1] PALOMO IRIGOYEN M, TAMAYO CARO M, PÉREZ ANDRÉS E, et al. Isolation and Purification of Primary Rodent Schwann Cells. Methods Mol Biol. 2018;1791:81-93. [2] SUGA M, HAYASHI Y, FURUE MK. In vitro models of cranial neural crest development toward toxicity tests: frog, mouse, and human. Oral Dis. 2017;23(5):559-565. [3] STRATTON JA, HOLMES A, ROSIN NL, et al. Macrophages Regulate Schwann Cell Maturation after Nerve Injury. Cell Rep. 2018;24(10):2561-2572.e6. [4] MIRFEIZI L, STRATTON JA, KUMAR R, et al. Serum-free bioprocessing of adult human and rodent skin-derived Schwann cells: implications for cell therapy in nervous system injury. J Tissue Eng Regen Med. 2017;11(12):3385-3397. [5] SUH JF, HYUNG S. Primary Motor Neuron Culture to Promote Cellular Viability and Myelination. Methods Mol Biol. 2018;1727: 403-411. [6] GERSEY ZC, BURKS SS, ANDERSON KD, et al. First human experience with autologous Schwann cells to supplement sciatic nerve repair: report of 2 cases with long-term follow-up. Neurosurg Focus. 2017;42(3):E2. [7] GORDON T, WOOD P, SULAIMAN OAR. Long-Term Denervated Rat Schwann Cells Retain Their Capacity to Proliferate and to Myelinate Axons in vitro. Front Cell Neurosci. 2019 ;12:511. [8] SHOJAPOUR M, MOSAYEBI G, HAJIHOSSEIN R, et al. A Simplified Protocol for the Purification of Schwann Cells and Exosome Isolation from C57BL/6 Mice. Rep Biochem Mol Biol. 2018;7(1):9-15. [9] WOOD PM. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976; 115(3):361-375. [10] BROCKES JP, FIELDS KL, RAFF MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165(1): 105-118. [11] ASKANAS V, ENGEL WK, DALAKAS MC, et al. Human schwann cells in tissue culture: histochemical and ultrastructural studies. Arch Neurol. 1980;37(6):329-337. [12] JIRSOVÁ K, SODAAR P, MANDYS V, et al. Cold jet: a method to obtain pure Schwann cell cultures without the need for cytotoxic, apoptosis-inducing drug treatment. J Neurosci Methods. 1997;78(1-2):133-137. [13] WU W, JIN YQ, KRETLOW JD, et al. Purification of Schwann cells from adult rats by differential detachment. Biotechnol Lett. 2009;31(11):1703-1708. [14] CASELLA GT, BUNGE RP, WOOD PM. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia. 1996;17(4): 327-338. [15] 王世清,范志海,沈忆新.不同部位取材的雪旺细胞培养与纯化研究[J].中国修复重建外科杂志,2011,25(2):166-170. [16] 秦煜,顾立强,吴岚晓,等.感觉性和运动性神经来源许旺细胞神经生长因子的表达[J].中华显微外科杂志,2001,24(4): 278-280. [17] HERCHER D, REDL H, SCHUH CMAP. Motor and sensory Schwann cell phenotype commitment is diminished by extracorporeal shockwave treatment in vitro. J Peripher Nerv Syst. 2020;25(1): 32-43. [18] TRACHTENBERG JT, THOMPSON WJ. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379(6561): 174-177. [19] LUTZ AB. Purification of schwann cells from the neonatal and injured adult mouse peripheral nerve. Cold Spring Harb Protoc. 2014;2014(12):1312-1319. [20] 汪海滨. 成年小鼠坐骨神经雪旺细胞体外培养的实验研究[D].广州:南方医科大学,2013. [21] 周敏, 胡鸣, 何松明, 等.新生大鼠施万细胞体外培养的实验研究[J].中国医药导报,2018,15(13):14-17,181. [22] TOMKO P, SLOVINSKÁ L, VANICKÝ I. In vitro predegeneration of peripheral nerve; the effect of predegeneration period on rat Schwann cell cultures. Exp Ther Med. 2019; 17(1):596-602. [23] WEI Y, ZHOU J, ZHENG Z, et al. An improved method for isolating Schwann cells from postnatal rat sciatic nerves. Cell Tissue Res. 2009;337(3):361-369. [24] JIN YQ, LIU W, HONG TH, et al. Efficient Schwann cell purification by differential cell detachment using multiplex collagenase treatment. J Neurosci Methods. 2008; 170(1):140-148. [25] ANDERSEN ND, MONJE PV. Isolation, Culture, and Cryopreservation of Adult Rodent Schwann Cells Derived from Immediately Dissociated Teased Fibers. Methods Mol Biol. 2018;1739:49-66. [26] ANDERSEN ND, SRINIVAS S, PIÑERO G, et al. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci Rep. 2016;6:31781. [27] NEEDHAM LK, TENNEKOON GI, MCKHANN GM. Selective growth of rat Schwann cells in neuron- and serum-free primary culture. J Neurosci. 1987;7(1):1-9. [28] KOMIYAMA T, NAKAO Y, TOYAMA Y, et al. A novel technique to isolate adult Schwann cells for an artificial nerve conduit. J Neurosci Methods. 2003;122(2):195-200. [29] HEDAYATPOUR A, SOBHANI A, BAYATI V, et al. A method for isolation and cultivation of adult Schwann cells for nerve conduit. Arch Iran Med. 2007;10(4):474-480. [30] 高德坤,孙辉,朱晋,等.乳鼠坐骨神经植块法的雪旺氏细胞培养[J].现代生物医学进展,2018,18(11):2028-2031,2037. [31] NIAPOUR A, KARAMALI F, KARBALAIE K, et al. Novel method to obtain highly enriched cultures of adult rat Schwann cells. Biotechnol Lett. 2010;32(6):781-786. [32] KRAUS A, TÄGER J, KOHLER K, et al. Efficacy of various durations of in vitro predegeneration on the cell count and purity of rat Schwann-cell cultures. J Neurotrauma. 2010;27(1):197-203. [33] HAASTERT-TALINI K. Culture and proliferation of highly purified adult Schwann cells from rat, dog, and man. Methods Mol Biol. 2012;846:189-200. [34] PIETRUCHA-DUTCZAKV M, MARCOL W, FRANCUZ T, et al. A new protocol for cultivation of predegenerated adult rat Schwann cells. Cell Tissue Bank. 2014; 15(3):403-411. [35] MILLER C, JEFTINIJA S, MALLAPRAGADA S. Micropatterned Schwann cell-seeded biodegradable polymer substrates significantly enhance neurite alignment and outgrowth. Tissue Eng. 2001;7(6):705-715. [36] SASAGASAKO N, TODA K, HOLLIS M, et al. Myelin gene expression in immortalized Schwann cells: relationship to cell density and proliferation. J Neurochem. 1996;66(4):1432-1439. [37] WANG H, WU J, ZHANG X, et al. Study of synergistic role of allogenic skin-derived precursor differentiated Schwann cells and heregulin-1β in nerve regeneration with an acellular nerve allograft. Neurochem Int. 2016;97:146-153. [38] FUENTEALBA L, SCHWORER C, SCHROERING A, et al. Heregulin and forskolin-induced cyclin D3 expression in Schwann cells: role of a CCAAT promoter element and CCAAT enhancer binding protein. Glia. 2004; 45(3): 238-248. [39] SOWA Y, KISHIDA T, TOMITA K, et al. Involvement of PDGF-BB and IGF-1 in Activation of Human Schwann Cells by Platelet-Rich Plasma. Plast Reconstr Surg. 2019;144(6):1025e-1036e. [40] 胡华麟,杨小四,杨元元,等.高糖及高糖加胰岛素对雪旺细胞培养的影响[J].包头医学院学报,2019,35(5):82-83. [41] KOMIYAMA A, SUZUKI K. Age-related changes in attachment and proliferation of mouse Schwann cells in vitro. Brain Res Dev Brain Res. 1991;62(1):7-16. [42] CHEN L, JIN Y, YANG X, et al. Fat tissue, a potential Schwann cell reservoir: isolation and identification of adipose-derived Schwann cells. Am J Transl Res. 2017; 9(5): 2579-2594. [43] SHEN M, TANG W, CAO Z, et al. Isolation of rat Schwann cells based on cell sorting. Mol Med Rep. 2017;16(2):1747-1752. [44] LIU Z, JIN YQ, CHEN L, et al. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS One. 2015; 10(4):e0123278. [45] TAO Y. Isolation and culture of Schwann cells. Methods Mol Biol. 2013;1018:93-104. [46] NIAPOUR N, MOHAMMADI-GHALEHBIN B, GOLMOHAMMADI MG, et al. Efficacy of optimized in vitro predegeneration period on the cell count and purity of canine Schwann cell cultures. Iran J Basic Med Sci. 2015;18(3):307-311. [47] ZHU J, QIN J, SHEN Z, et al. Dispase rapidly and effectively purifies Schwann cells from newborn mice and adult rats. Neural Regen Res. 2012;7(4):256-260. [48] ANAND U, OTTO WR, BOUNTRA C, et al. Cytosine arabinoside affects the heat and capsaicin receptor TRPV1 localisation and sensitivity in human sensory neurons. J Neurooncol. 2008;89(1):1-7. [49] VROEMEN M, WEIDNER N. Purification of Schwann cells by selection of p75 low affinity nerve growth factor receptor expressing cells from adult peripheral nerve. J Neurosci Methods. 2003;124(2):135-143. [50] SCHUH CM, HAUSNER T, REDL HR. A therapeutic shock propels Schwann cells to proliferate in peripheral nerve injury. Brain Circ. 2016;2(3):138-140. [51] MITTERMAYR R, HARTINGER J, ANTONIC V, et al. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann Surg. 2011;253(5):1024-1032. [52] HAUSNER T, PAJER K, HALAT G, et al. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp Neurol. 2012;236(2):363-370. [53] SCHUH CM, HERCHER D, STAINER M, et al. Extracorporeal shockwave treatment: A novel tool to improve Schwann cell isolation and culture. Cytotherapy. 2016;18(6): 760-770. [54] WEIHS AM, FUCHS C, TEUSCHL AH, et al. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014;289(39):27090-27104. [55] HAYNES LW, RUSHTON JA, PERRINS MF, et al. Diploid and hyperdiploid rat Schwann cell strains displaying negative autoregulation of growth in vitro and myelin sheath-formation in vivo. J Neurosci Methods. 1994;52(2):119-127. [56] BROOKS AE, ATHAUDA G, BUNGE MB, et al. Culture and Expansion of Rodent and Porcine Schwann Cells for Preclinical Animal Studies. Methods Mol Biol. 2018;1739: 111-126. [57] WU Z, LI Q, XIE S, et al. In vitro and in vivo biocompatibility evaluation of a 3D bioprinted gelatin-sodium alginate/rat Schwann-cell scaffold. Mater Sci Eng C Mater Biol Appl. 2020;109:110530. [58] 张振辉,王庆德,梅伟,等.新型组织工程化神经导管修复大鼠周围神经缺损[J].中华显微外科杂志,2018,41(6):563-567. [59] STRAUCH B, RODRIGUEZ DM, DIAZ J, et al. Autologous Schwann cells drive regeneration through a 6-cm autogenous venous nerve conduit. J Reconstr Microsurg. 2001;17(8):589-595. [60] CHEN W, WEI ZR, WU BH, et al. Effects of combined transplantation of rat Schwann cells and fibroblasts on nerve regeneration of denervated perforator flaps in rats and the mechanism. Zhonghua Shao Shang Za Zhi. 2019;35(2):134-142. [61] SAHEB-AL-ZAMANI M, YAN Y, FARBER SJ, et al. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol. 2013; 247:165-177. [62] LEVI AD, BURKS SS, ANDERSON KD, et al. The Use of Autologous Schwann Cells to Supplement Sciatic Nerve Repair With a Large Gap: First in Human Experience. Cell Transplant. 2016;25(7):1395-1403. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [5] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [6] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [7] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [8] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [9] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [10] | Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling. Photoreceptor cell replacement therapy for retinal degeneration diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1116-1121. |
| [11] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| [12] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [13] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| [14] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [15] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||