Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (7): 1142-1148.doi: 10.3969/j.issn.2095-4344.2183
Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis
Kong Desheng1, 2, He Jingjing1, 2, Feng Baofeng1, 2, Guo Ruiyun1, 2, Asiamah Ernest Amponsah1, 2, Lü Fei1, 2, Zhang Shuhan1, 2, Zhang Xiaolin3, Ma Jun1, 2, 4, Cui Huixian1, 2, 4
- 1Hebei Medical University-National University of Ireland Galway Stem Cell Research Center, Shijiazhuang 050017, Hebei Province, China; 2Hebei Research Center for Stem Cell Medical Translational Engineering, Shijiazhuang 050017, Hebei Province, China; 3Department of Epidemiology and Health Statistics, 4Department of Anatomy, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China
-
Received:2020-04-20Revised:2020-04-23Accepted:2020-05-28Online:2021-03-08Published:2020-12-09 -
Contact:Ma Jun, MD, Associate professor, Hebei Medical University-National University of Ireland Galway Stem Cell Research Center, Shijiazhuang 050017, Hebei Province, China; Hebei Research Center for Stem Cell Medical Translational Engineering, Shijiazhuang 050017, Hebei Province, China; Department of Anatomy, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China Cui Huixian, MD, Professor, Hebei Medical University-National University of Ireland Galway Stem Cell Research Center, Shijiazhuang 050017, Hebei Province, China; Hebei Research Center for Stem Cell Medical Translational Engineering, Shijiazhuang 050017, Hebei Province, China; Department of Anatomy, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China -
About author:Kong Desheng, MD, Hebei Medical University-National University of Ireland Galway Stem Cell Research Center, Shijiazhuang 050017, Hebei Province, China; Hebei Research Center for Stem Cell Medical Translational Engineering, Shijiazhuang 050017, Hebei Province, China -
Supported by:the High-Level Talent Funding Project in Hebei Province, No. B2019005009; the Youth Program of National Natural Science Foundation of China, No. 81801278; the General Program of Natural Science Foundation of Hebei Province, No. H2019206637
CLC Number:
Cite this article
Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148.
share this article
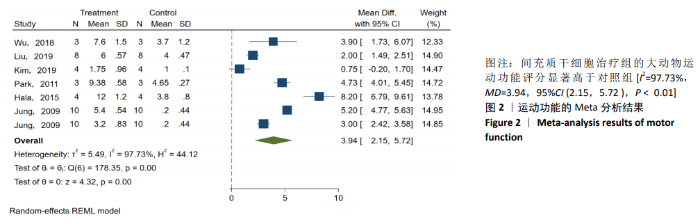
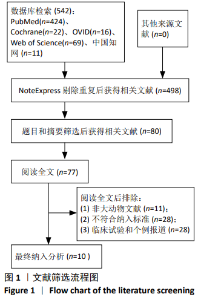
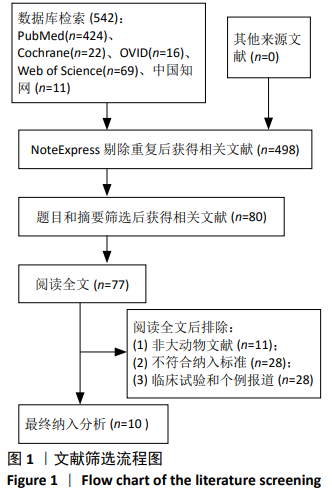
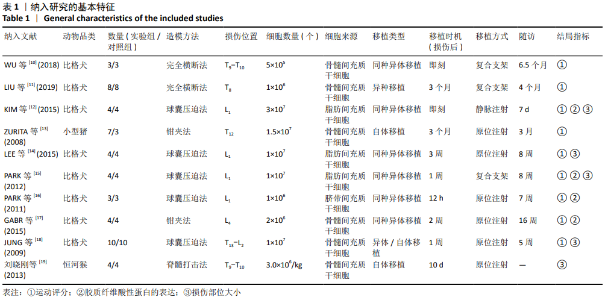
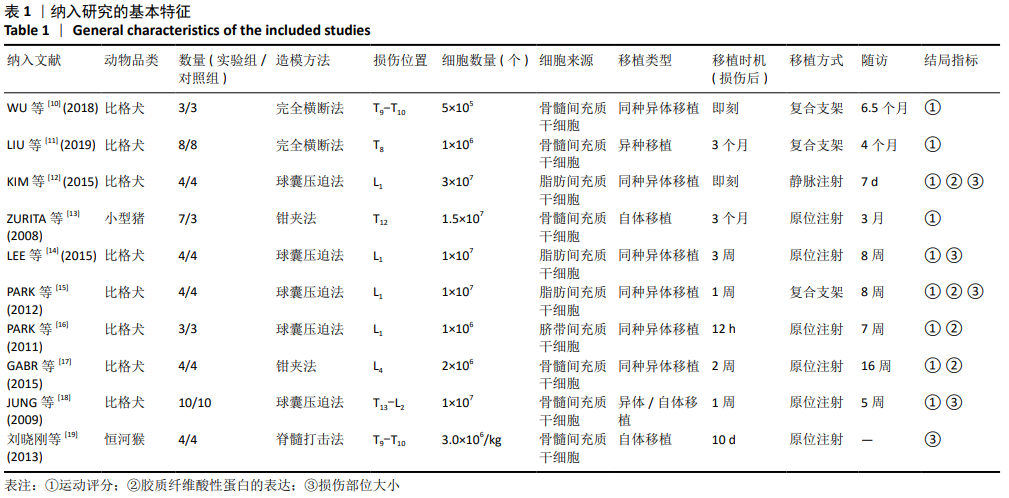
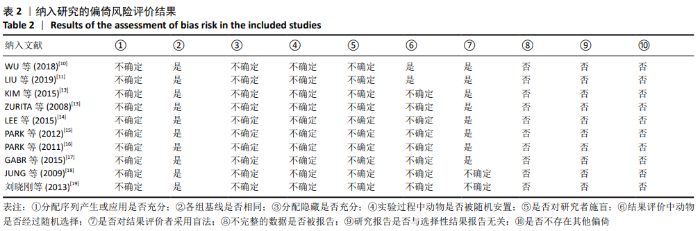
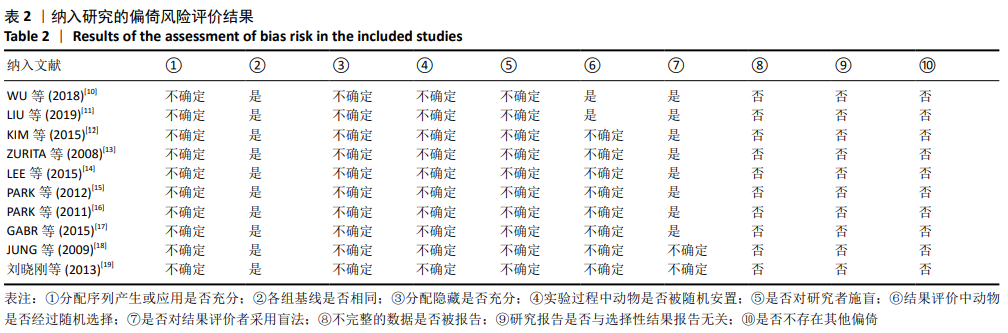
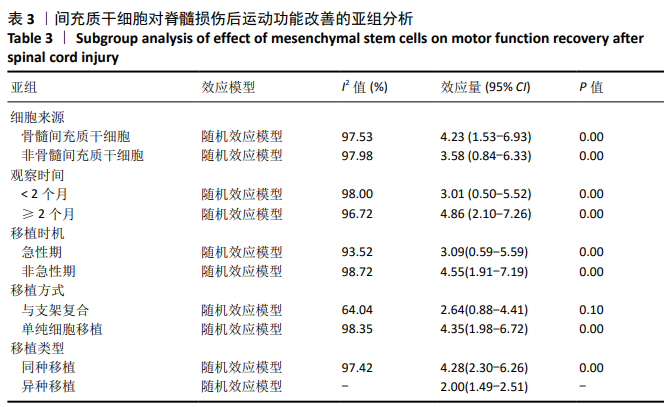
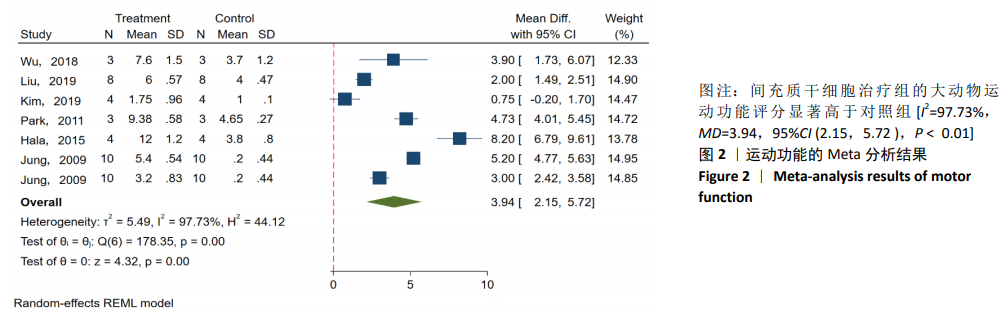
2.3 Meta分析结果 2.3.1 运动功能的Meta分析 5个研究报告了脊髓损伤后间充质干细胞对大动物运动功能的影响[10-12,16,18],运用Olby评分表进行评价,其中1个研究包含自体移植和异体移植2个实验 组[18],因此对运动功能的分析包括6个研究,合计标本量为84只动物,实验组和对照组各42只。各研究结果之间异质性较高,选择随机效应模型,结果显示间充质干细胞治疗组的运动评分明显高于对照组[I2=97.73%,MD=3.94,95%CI (2.15,5.72 ),P < 0.01],见图2。根据不同细胞来源、观察时间、移植时机、移植方式和移植类型分不同的亚组进行分析。结果显示:①在细胞来源方面,骨髓间充质干细胞和非骨髓间充质干细胞来源组的运动评分均明显高于对照组,差异有显著性意义,MD=4.23和3.58,95%CI分别为1.53-6.93,0.84-6.33;②在观察时间方面,短时间观察(< 2个月)和长时间观察(≥2个月)的间充质干细胞治疗组的运动功能评分均高于对照组;③在大动物模型急性期进行移植组运动评分高于对照组[MD=3.09,95%CI (0.59-5.59),P < 0.01],同样,在非急性期移植组也有相似结果,运动评分明显高于对照组[MD=4.55,95%CI (1.91-7.19),P < 0.01];④与支架复合移植组运动评分相较对照组,差异无显著性意义[MD=2.64,95%CI (0.88-4.41),P=0.10],而单纯细胞移植组的运动评分明显高于对照组[MD=4.35,95%CI(1.98-6.72),P < 0.01];⑤在移植类型方面,因5个研究为异种移植,异种移植组的运动评分高于对照组[MD=4.28,95%CI (2.30-6.26),P < 0.01],而同种移植只有1项研究,无其他统计结果。具体数据详见表3。"
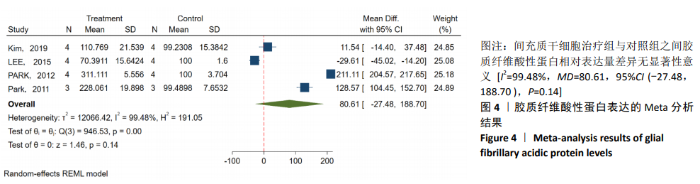
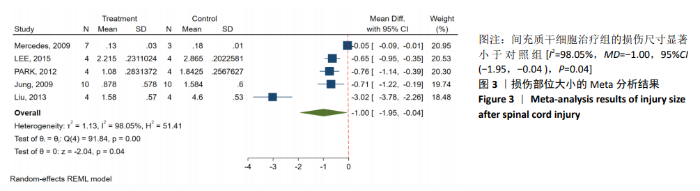
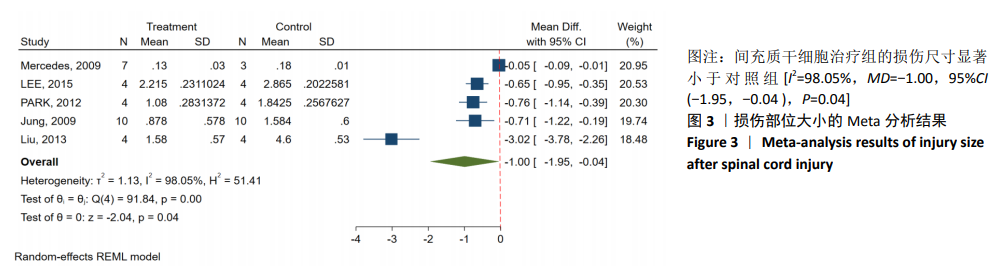
2.3.3 胶质纤维酸性蛋白表达水平的Meta分析 4个研究报告了间充质干细胞对大动物脊髓损伤后组织中胶质纤维酸性蛋白的表达[12,14-16],运用Western blot或PCR检测损伤部位中胶质纤维酸性蛋白的相对表达量。合计标本量为30只动物,实验组和对照组分别为15只。各研究结果之间异质性较高,选择随机效应模型,结果显示间充质干细胞治疗组与对照组之间胶质纤维酸性蛋白相对表达量差异无显著性意义[I2=99.48%,MD=80.61,95%CI (-27.48,188.70 ),P=0.14],见图4。 2.3.4 发表偏倚的检测 运动功能分析中纳入6个研究,发表偏倚检验结果:t=0.60,P=0.546,不存在发表偏倚;损伤部位大小分析中纳入5个研究,发表偏倚检验结果:t=-3.3,P=0.046,存在发表偏倚;胶质纤维酸性蛋白表达水平分析中纳入4个研究,发表偏倚检验结果:t=-0.81,P=0.501,不存在发表偏倚。 2.3.5 敏感性分析 对异质性大的结局指标通过逐一删除研究、转换效应模型等方法进行敏感性分析,I2差别不明显,考虑与研究纳入模型的疾病严重程度、方法学等方面相关。 "

| [1] BADHIWALA JH, WILSON JR, FEHLINGS MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019;18(1): 24-25. [2] GBD 2016 TRAUMATIC BRAIN INJURY AND SPINAL CORD INJURY COLLABORATORS. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56-87. [3] 刘克勋,霍洪军,赵岩,等. 间充质干细胞移植治疗脊髓损伤的进展及发展趋势[J]. 中国组织工程研究,2018,22(21): 3410-3416. [4] NITZSCHE F, MÜLLER C, LUKOMSKA B, et al. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells. 2017;35(6): 1446-1460. [5] MUKHAMEDSHINA YO, AKHMETZYANOVA ER, KOSTENNIKOV AA, et al. Adipose-Derived Mesenchymal Stem Cell Application Combined With Fibrin Matrix Promotes Structural and Functional Recovery Following Spinal Cord Injury in Rats. Front Pharmacol. 2018;9:343. [6] COFANO F, BOIDO M, MONTICELLI M, et al. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int J Mol Sci. 2019;20(11):2698. [7] KWON BK, STREIJGER F, HILL CE, et al. Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp Neurol. 2015;269:154-168. [8] ALIZADEH A, DYCK SM, KARIMI-ABDOLREZAEE S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019;10:282. [9] HOOIJMANS CR, ROVERS MM, DE VRIES RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [10] WU GH, SHI HJ, CHE MT, et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials. 2018;181: 15-34. [11] LIU D, LI X, XIAO Z, et al. Different functional bio-scaffolds share similar neurological mechanism to promote locomotor recovery of canines with complete spinal cord injury. Biomaterials. 2019;214:119230. [12] KIM Y, JO SH, KIM WH, et al. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res Ther. 2015;6:229. [13] ZURITA M, VAQUERO J, BONILLA C, et al. Functional recovery of chronic paraplegic pigs after autologous transplantation of bone marrow stromal cells. Transplantation. 2008;86(6):845-853. [14] LEE SH, KIM Y, RHEW D, et al. Effect of the combination of mesenchymal stromal cells and chondroitinase ABC on chronic spinal cord injury. Cytotherapy. 2015;17(10): 1374-1383. [15] PARK SS, LEE YJ, LEE SH, et al. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy. 2012;14(5):584-597. [16] PARK SS, BYEON YE, RYU HH, et al. Comparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotrophin-3. Cell Transplant. 2011;20(11-12):1867-1880. [17] GABR H, EL-KHEIR WA, FARGHALI HA, et al. Intrathecal Transplantation of Autologous Adherent Bone Marrow Cells Induces Functional Neurological Recovery in a Canine Model of Spinal Cord Injury. Cell Transplant. 2015;24(9):1813-1827. [18] JUNG DI, HA J, KANG BT, et al. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci. 2009;285(1-2):67-77. [19] 刘晓刚,邓宇斌,蔡辉,等. 控释胶质细胞源性神经营养因子与骨髓间充质干细胞源神经元样细胞移植可减少脊髓损伤后空洞形成[J].中国组织工程研究,2013,17(1):68-73. [20] BASSO DM, FISHER LC, ANDERSON AJ, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23(5):635-659. [21] BASSO DM, BEATTIE MS, BRESNAHAN JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1-21. [22] YANG Z, WANG KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38(6):364-374. [23] ROPPER AE, THAKOR DK, HAN I, et al. Defining recovery neurobiology of injured spinal cord by synthetic matrix-assisted hMSC implantation. Proc Natl Acad Sci U S A. 2017;114(5):E820-E829. [24] NAKAJIMA H, UCHIDA K, GUERRERO AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29(8):1614-1625. [25] SHIUE SJ, RAU RH, SHIUE HS, et al. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain. 2019;160(1):210-223. [26] ABBASZADEH HA, NIKNAZAR S, DARABI S, et al. Stem cell transplantation and functional recovery after spinal cord injury: a systematic review and meta-analysis. Anat Cell Biol. 2018;51(3):180-188. [27] FU H, HU D, ZHANG L, et al. Efficacy of Oligodendrocyte Progenitor Cell Transplantation in Rat Models with Traumatic Thoracic Spinal Cord Injury: A Systematic Review and Meta-Analysis. J Neurotrauma. 2018;35(21):2507-2518. [28] PAPA S, VISMARA I, MARIANI A, et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J Control Release. 2018;278:49-56. [29] SUN G, LI G, LI D, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194-204. [30] RYU HH, KANG BJ, PARK SS, et al. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J Vet Med Sci. 2012;74(12):1617-1630. [31] YOUSEFIFARD M, NASSERI MALEKI S, ASKARIAN-AMIRI S, et al. A combination of mesenchymal stem cells and scaffolds promotes motor functional recovery in spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. 2019;32(2):269-284. [32] 潘晓明,吴玲玲,陈浩浩,等. 脐带间充质干细胞移植对脊髓损伤小鼠运动功能的修复和镇痛作用[J]. 解剖学杂志, 2020,43(1):3-9. [33] KRUPA P, VACKOVA I, RUZICKA J, et al. The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. Int J Mol Sci. 2018;19(5):1503. [34] LI G, CHE MT, ZHANG K, et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233-248. [35] TAKAHASHI A, NAKAJIMA H, UCHIDA K, et al. Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury. Cell Transplant. 2018; 27(7):1126-1139. [36] MUNISWAMI DM, KANTHAKUMAR P, KANAKASABAPATHY I, et al. Motor Recovery after Transplantation of Bone Marrow Mesenchymal Stem Cells in Rat Models of Spinal Cord Injury. Ann Neurosci. 2019; 25(3):126-140. [37] LIU AM, CHEN BL, YU LT, et al. Human adipose tissue- and umbilical cord-derived stem cells: which is a better alternative to treat spinal cord injury? Neural Regen Res. 2020;15(12):2306-2317. [38] TIAN DZ, DENG D, QIANG JL, et al. Repair of spinal cord injury in rats by umbilical cord mesenchymal stem cells through P38MAPK signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(3 Suppl):47-53. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Min Youjiang, Yao Haihua, Sun Jie, Zhou Xuan, Yu Hang, Sun Qianpu, Hong Ensi. Effect of “three-tong acupuncture” on brain function of patients with spinal cord injury based on magnetic resonance technology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-8. |
| [3] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [4] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [5] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [6] | Chen Jinping, Li Kui, Chen Qian, Guo Haoran, Zhang Yingbo, Wei Peng. Meta-analysis of the efficacy and safety of tranexamic acid in open spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1458-1464. |
| [7] | Hu Kai, Qiao Xiaohong, Zhang Yonghong, Wang Dong, Qin Sihe. Treatment of displaced intra-articular calcaneal fractures with cannulated screws and plates: a meta-analysis of 15 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1465-1470. |
| [8] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [9] | Wang Yongsheng, Wu Yang, Li Yanchun. Effect of acute high-intensity exercise on appetite hormones in adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1305-1312. |
| [10] | Jiang Hongying, Zhu Liang, Yu Xi, Huang Jing, Xiang Xiaona, Lan Zhengyan, He Hongchen. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1149-1153. |
| [11] | Wu Xun, Meng Juanhong, Zhang Jianyun, Wang Liang. Concentrated growth factors in the repair of a full-thickness condylar cartilage defect in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1166-1171. |
| [12] | Shen Jinbo, Zhang Lin. Micro-injury of the Achilles tendon caused by acute exhaustive exercise in rats: ultrastructural changes and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1190-1195. |
| [13] | Li Jing, Xie Jianshan, Cui Huilin, Cao Ximei, Yang Yanping, Li Hairong. Expression and localization of diacylglycerol kinase zeta and protein kinase C beta II in mouse back skin with different coat colors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1196-1200. |
| [14] | Chai Le, Lü Jianlan, Hu Jintao, Hu Huahui, Xu Qingjun, Yu Jinwei, Quan Renfu. Signal pathway variation after induction of inflammatory response in rats with acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1218-1223. |
| [15] | Tan Jingyu, Liu Haiwen. Genome-wide identification, classification and phylogenetic analysis of Fasciclin gene family for osteoblast specific factor 2 [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1243-1248. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||