Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (34): 5441-5446.doi: 10.3969/j.issn.2095-4344.2312
Previous Articles Next Articles
Application of nanocomposite hydrogels in bone tissue engineering
Yu Xingge1, Lin Kaili2
1School&Hospital of Stomatology, Tongji University, Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Shanghai 200072, China; 2Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China
-
Received:2019-12-02Revised:2019-12-04Accepted:2020-01-18Online:2020-11-08Published:2020-09-11 -
Contact:Lin Kaili, Professor, Doctoral supervisor, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China -
About author:Yu Xingge, Doctoral candidate, School&Hospital of Stomatology, Tongji University, Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Shanghai 200072, China -
Supported by:National Key Research and Development Project, No. 2018YFB1105605 ; the National Natural Science Foundation of China, No. 81871490
CLC Number:
Cite this article
Yu Xingge, Lin Kaili. Application of nanocomposite hydrogels in bone tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5441-5446.
share this article
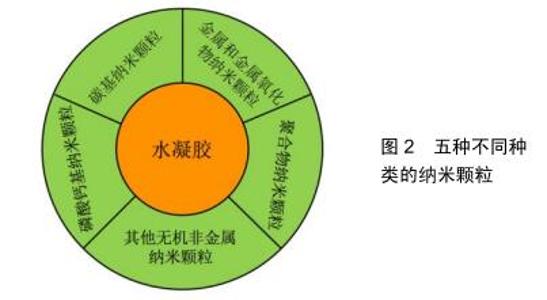
水凝胶结构链上通常含有大量的活性官能团,这些官能团可以与纳米颗粒发生物理或化学交联,从而改变水凝胶的物理、化学及生物特性。根据纳米颗粒的物理化学特性,将常用于骨组织工程的纳米颗粒分为以下5种,见图2。 2.1 碳基纳米颗粒 常见的碳基纳米颗粒有碳纳米管、石墨烯、富勒烯、纳米金刚石等。其中作为复合材料的纳米填料,碳纳米管是最常见、也是被认为最优秀的纳米颗粒。碳纳米管是一种具有特殊结构的一维量子材料,主要由呈六边形排列的碳原子构成数层到数十层的同轴圆管。碳纳米管可以看作是石墨烯片层卷曲而成,按照石墨烯片的层数可分为单壁碳纳米管、双壁碳纳米管和多壁碳纳米管[12]。双壁碳纳米管和多壁碳纳米管有两层或多层石墨烯片层组成,片层之间间隔约为0.34 nm。碳纳米管具有较高的柔韧性、较低的质量密度和较大的比表面积,并且它还具有出色的热、电和机械性能[13],这些独特的性质使其在许多方面得到了应用,如传感器、太阳能电池、药物递送、组织工程等[14-15]。而碳纳米管在成骨诱导方面的功能也已经得到验证[16-17]。 然而,碳纳米管在水凝胶中具有不溶性和聚集性,限制了它作为纳米填料的应用。因此,目前对碳纳米管进行了多种类型的改性研究(包括共价和非共价改性)来改善它的分散性,并与周围水凝胶建立良好的交联作用。非共价改性包括表面活性剂溶解、聚合物包裹和聚合物吸收。而更常见的是碳纳米管的共价官能化,主要通过在其表面添加不同类型的官能团来实现。 以碳纳米管为纳米填料改性的水凝胶在骨组织工程中有非常大的潜力。ZHANG等[18]利用了一种具有特殊表面化学和仿生特征的玫瑰花样碳纳米管,这种新型碳纳米管在水溶液中自组装形成直径约4 nm的稳定纳米管结构,其仿生纳米结构可以促进成骨细胞的黏附,同时还是优良的骨基质钙化模板[19-20]。因此他们将玫瑰花样碳纳米管添加到聚甲基丙烯酸羟乙酯水凝胶中,并用RGD肽进行表面修饰,研究表明这种玫瑰花样碳纳米管-聚甲基丙烯酸羟乙酯复合水凝胶促进了成骨细胞的黏附和增殖,是一种具有潜力的骨组织工程材料。CUI等[21]将具有成骨诱导活性的多壁碳纳米管与高强度的聚合物离子水凝胶进行结合,利用3D打印技术制造纳米复合水凝胶骨修复支架,以期为体内的骨再生提供一个合适的环境。研究表明,这种纳米复合水凝胶骨修复支架的机械强度高、生物相容性好,同时骨髓间充质干细胞在这个支架上还表现出良好的成骨、成血管分化能力。这一结论在SD大鼠颅骨缺损模型的修复上也同样得到了验证。 2.2 金属和金属氧化物纳米颗粒 通常水凝胶结构链上拥有丰富的羟基、羧基等官能团,这些官能团很容易与金属或金属氧化物发生离子反应而结合。最常作为纳米填料的金属有金[22]、银[23]、钙[24]、钡等[24],金属氧化物则有氧化铁[25]、氧化镁等[26]。众所周知,骨骼是一种弱压电材料,机电效应参与了骨骼的改建与重塑。而金属和金属氧化物纳米颗粒通常都拥有优越的导电性和磁性,它们的掺入既可以增强水凝胶的机械强度,还有可能通过导电性和磁性作用促进骨再生。JAISWAL等[25]设计了一种掺入超低浓度Fe3O4磁性纳米颗粒的增强型复合甲基丙烯酰基明胶水凝胶,这种复合水凝胶的压缩模量增加了10倍以上,韧性增加了20倍以上。人骨髓间充质干细胞不仅在该水凝胶中有90%以上的存活率,同时还能感知机械信号,表现为细胞不同的铺展面积和伪足,可能可以促进其成骨分化。HEO等[22]构建了可生物降解的甲基丙烯酰基明胶水凝胶支架,并负载了金纳米颗粒,结果表明这种复合水凝胶支架能促进人脂肪间充质干细胞的增殖、分化和碱性磷酸酶活性,从而向成骨细胞分化。 2.3 聚合物纳米颗粒 与碳基纳米颗粒、金属及金属氧化物纳米颗粒相比,聚合物纳米颗粒具有良好的生物可降解性能,这在再生医学中有着巨大的优势。聚合物纳米颗粒的形式主要有纳米脂质体、树枝状聚合物、聚合物胶束、纳米凝胶等[27]。脂质体是连续的、封闭的圆形囊泡,由分散在水性介质的一层或多层磷脂双层形成。由于磷脂的两亲性,纳米脂质体被广泛地应用于药物及基因递送和组织工程[28-31]。树枝状聚合物是高度分支的对称聚合物分子,由一个内部核及多层树枝状分子重复单元组成。由于它表面有丰富的活性基团,对其外部基团进行简单的官能化就能用来开发载有药物的生物材料[32],同时在组织修复中也有很广泛的应用前景[33-34]。聚合物胶束是一种两性聚合物的自组装体,它通过自组装形成嵌段共聚物的核-壳胶束结构(疏水性核和亲水性壳)。由于它的两亲性、体积小、药物缓释、所携带药物水溶性增强等特点,聚合物胶束是疏水药物的理想载 体[35]。纳米凝胶是纳米级交联的凝胶颗粒,可以通过非共价键进行物理交联,也可以通过共价键进行化学交联。由于纳米凝胶的稳定性、生物相容性、水溶性及药物缓释能力,纳米凝胶已在药物输送载体和组织工程支架领域中得到广泛的研究和应用[36-40]。 聚合物纳米颗粒的掺入主要有以下2个功能:首先是增强水凝胶的机械性能。骨植入材料模拟天然组织的弹性模量至关重要,皮质骨和松质骨的平均弹性模量分别为5.44 GPa和4.59 GPa,而水凝胶的低弹性模量明显限制了其在骨再生中的应用。RAHALI等[41]用天然衍生的纳米脂质体对甲基丙烯酰基明胶水凝胶进行功能化,不仅增强了甲基丙烯酰基明胶水凝胶的机械性能,还表现出较高的抗扭曲和剪切强度。XIAO等[42]开发了一种两亲性嵌段共聚物,它可以自组装为直径21 nm的胶束,并将其掺入聚丙烯酰胺水凝胶中。通过改变嵌段共聚物胶束的浓度调控水凝胶的机械性能,当胶束质量浓度从7.5 g/L增加至15 g/L时,杨氏模量增加了4倍,拉伸应力增加了2倍。再者,这些聚合物纳米颗粒都是非常优良的药物和生物活性因子运送载体,既能保障药物和生物活性因子的活性又能控制其释放。FUJIOKA-KOBAYASHI等[43]开发了一种胆固醇和丙烯酰基修饰的支链淀粉形成的纳米凝胶,并在其内部装载了骨形态发生蛋白2和重组人纤维细胞生长因子18,并将这种纳米凝胶与硫醇基改性的聚乙二醇共交联形成可生物降解的纳米复合水凝胶。动物实验表明,这种胆固醇和丙烯酰基修饰的支链淀粉/水凝胶材料作为骨形态发生蛋白2和纤维细胞生长因子18的载体,可调节因子的释放来诱导成骨。除药物递送外,SAMADIKUCHAKSARAEI等[44]将纳米脂质体作为纳米羟基磷灰石的成核位点,这种纳米羟基磷灰石/明胶复合水凝胶适宜成骨细胞的黏附,还显示出良好的生物相容性、生物可降解性及骨传导性能。 2.4 磷酸钙基纳米颗粒 磷酸钙与哺乳动物正常钙化组织(骨骼、牙齿)的无机成分组成相似,具有优越的生物相容性、骨传导性,并且可以直接与骨进行结合,因而受到了广泛的关注[45-46]。组织工程中常用的磷酸钙是β-磷酸三钙和羟基磷灰石。β-磷酸三钙是磷酸钙的高温相,只有在800 ℃以上的温度下才能通过热分解获得。羟基磷灰石在pH值高于4.2的水溶液中能稳定存在,是磷酸钙中溶解性最差的一类结晶化合物。羟基磷灰石可以通过湿法制备[47],如沉淀法[48]、水热法或其他磷酸钙水解转化法等,同时还可以在1 200 ℃以上的温度下由一水合磷酸氢钙、无水磷酸一钙、磷酸氢钙二水化合物等与氧化钙、氢氧化钙或碳酸钙的固相反应获得。尽管β-磷酸三钙与羟基磷灰石在化学成分上相似,但它们的生物可降解能力却明显不同。羟基磷灰石在生物体内的吸收很慢,植入体内后,羟基磷灰石可能会整合到再生的骨组织中,而β-磷酸三钙则会被完全吸收[49]。实际运用过程中,可降解的β-磷酸三钙可以与羟基磷灰石结合运用,从而调控材料的降解速率与新骨的形成速率相匹配。 正常生物体骨组织中除了Ca和P,还有一些其他元素,如镁、锶、锌、钾等。因此,已有研究表明这些元素的掺杂可以使磷酸钙材料表现出更好的骨再生行为。镁在骨骼组织中高度富集,并表现出对矿物代谢的间接影响[50];锶被认为具有减少骨吸收和促进骨再生的能 力[51];锌能促进成骨细胞的增殖和分化[52];钾在调节生化过程中有多种用途,并在磷灰石矿物成核过程中起着重要作用[53];钠在细胞黏附及骨代谢中有潜在作用[54]。除此之外,一些其他元素的掺杂也能起到积极的作用。银的掺杂可以提高骨植入物的抗菌能力[55];锰掺杂可以促进MC3T3成骨细胞样细胞的体外增殖和成骨分化[56];硅掺杂可以增强成骨细胞的黏附、迁移、增殖和分化[57]。 由于磷酸钙基纳米颗粒有较好的机械性能和骨传导性能[58],因此在纳米复合水凝胶中的贡献主要在于提高水凝胶机械强度及骨传导性能。FU等[59]合成了一种聚乙二醇-聚己内酯-聚乙二醇三嵌段共聚物/胶原/纳米羟基磷灰石可注射温敏复合水凝胶,在大鼠肌肉袋中表现出良好的生物相容性和生物可降解性,同时在新西兰白兔的颅骨缺损实验中实现了良好的骨再生。BUENO等[60]在黄胶原的水溶液中混入纳米羟基磷灰石和锶掺杂纳米羟基磷灰石,并利用柠檬酸作为交联剂形成纳米复合水凝胶。黄胶原水凝胶结构链与纳米颗粒的复合增强了水凝胶的机械性能,同时纳米羟基磷灰石和锶掺杂纳米羟基磷灰石还能提高成骨细胞的碱性磷酸酶活性,锶掺杂纳米羟基磷灰石组拥有更好的促成骨效果。除此之外,磷酸钙基纳米颗粒还可作为药物载体及生物活性因子载体,与水凝胶结合作为一种缓释系统。WU等[61]利用模板法制备了介孔羟基磷灰石,并装载了可诱导骨再生的药物——辛伐他汀,混入聚N-异丙基丙烯酰胺温敏水凝胶中。这种纳米复合水凝胶可以在37 ℃下保持16 d持续缓慢释放辛伐他汀,并且能促进骨髓间充质干细胞的黏附和成骨分化。 2.5 其他无机非金属纳米颗粒 除了磷酸钙基纳米颗粒外,其他无机非金属纳米颗粒也常被用来作为促进骨再生的纳米填料,如介孔二氧化硅纳米颗粒[62]、介孔生物活性玻璃纳米颗粒[63]、黏土纳米颗粒等[64]。 介孔二氧化硅纳米颗粒表面具有丰富的羟基官能团,这些官能团能与海藻酸钠、明胶等水凝胶的羧基形成交联,以此来提高水凝胶的机械性能和黏度。而介孔二氧化硅纳米颗粒不仅可以促进骨传导,改善成骨细胞增殖并诱导成骨分化[65],同时其释放的硅组分还可以增强内皮细胞的血管生成能力[66]。此外,介孔二氧化硅纳米颗粒的高比表面积和介孔孔道还具有装载各类药物和生物活性因子的功能。这些都体现了介孔二氧化硅纳米颗粒作为纳米填料应用于骨组织工程的巨大优势。在最近的一项研究中,ROOPAVATH等[62]开发了一种SiO2纳米颗粒-海藻酸钠-明胶复合水凝胶,不仅改善了水凝胶的压缩模量和黏度,还促进了人脐带间充质干细胞的黏附和成骨分化能力。最后他们还扫描重建了患者的骨缺损区域,通过3D打印的方法构建了个性化骨移植物来验证其可打印性,显示了这种纳米复合水凝胶在骨组织工程中的巨大潜力。 生物活性玻璃是由HENCH教授在20世纪70年代首先合成的,一般为SiO2-CaO-P2O5系统的玻璃,也可以在玻璃网络结构中掺杂Na2O、MgO、Al2O3等组分。生物活性玻璃在模拟体液或生物体内环境易降解并诱导碳酸代类骨磷灰石沉积,并与宿主骨组织形成键合。而介孔生物活性玻璃具有更高的比表面积、均一的介孔分布及更大的孔容,因此与传统生物活性玻璃相比,介孔生物活性玻璃拥有更快的诱导碳酸代类骨磷灰石沉积速率、更好的生物活性。XIN等[63]对介孔生物活性玻璃纳米颗粒进行甲基丙烯酰基明胶的化学修饰,使其能与甲基丙烯酰基明胶水凝胶形成无机/有机共交联水凝胶。与介孔生物活性玻璃直接掺入甲基丙烯酰基明胶水凝胶相比,无机/有机共交联水凝胶拥有更好的机械性能、持久的降解时间和更优越的矿化性能。研究表明,无 机/有机共交联水水凝胶在体外有利于细胞的黏附、增殖及成骨分化,同时在体内具有促进血管生成和骨再生的能力,进而加速SD大鼠临界颅骨缺损的修复与再生。 近年来,黏土纳米颗粒和水凝胶的复合材料引起学者的关注。首先,黏土纳米颗粒与水凝胶的复合可以增强其机械性能;其次,黏土纳米颗粒在携带药物和生物活性因子方面也得到了广泛的研究和验证[67];最后,黏土纳米颗粒和水凝胶的复合材料在调控细胞黏附、增殖和成骨分化方面也取得了一系列进展。KERATIVITAYANAN等[68]利用纳米锂皂石与可生物降解的聚癸二酸甘油酯复合制备了刚度可调的多孔弹性纳米复合材料支架。这种新型纳米复合支架能促进细胞的黏附、扩散和增殖,提高碱性磷酸酶表达,增加骨基质的矿化,体内研究还验证了其生物相容性和生物可降解性。 "
|
[1] EINHORN TA, GERSTENFELD LC. Fracture healing: mechanisms and interventions.Nat Rev Rheumatol. 2015;11(1):45-54.
[2] BOSE S, ROY M, BANDYOPADHYAY A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol.2012;30(10):546-554.
[3] AMINI AR, LAURENCIN CT, NUKAVARAPU SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363-408.
[4] HENKEL J, WOODRUFF MA, EPARI DR, et al. Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective. Bone Res.2013;1:216-248.
[5] PINA S,OLIVEIRA JM, REIS RL. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review.Adv Mater.2015;27(7):1143-1169.
[6] SPRIO S, RUFFINI A, VALENTINI F, et al. Biomimesis and biomorphic transformations: New concepts applied to bone regeneration.J Biotechnol. 2011;156(4):347-355.
[7] GEORGIADIS M, MULLER R, SCHNEIDER P. Techniques to assess bone ultrastructure organization: orientation and arrangement of mineralized collagen fibrils.J R Soc Interface.2016;13(119):1-26.
[8] BURGER C, ZHOU HW, WANG H, et al. Lateral packing of mineral crystals in bone collagen fibrils. Biophys J.2008;95(4):1985-1992.
[9] GAHARWAR AK, PEPPAS NA, KHADEMHOSSEINI A. Nanocomposite Hydrogels for Biomedical Applications. Biotechnol Bioeng.2014;111(3):441-453.
[10] 康肸,邓爱鹏,杨树林.壳聚糖基温敏水凝胶的研究进展[J].中国生物工程杂志,2018,38(5):79-84.
[11] WANG Q, MYNAR JL, YOSHIDA M, et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature. 2010;463(7279):339-343.
[12] SAJID MI, JAMSHAID U, JAMSHAID T, et al. Carbon nanotubes from synthesis to in vivo biomedical applications.Int J Pharm. 2016;501(1-2): 278-299.
[13] LIU ZH, YANG XF, WANG GS, et al. Influence of carbon nanotube suspension on the thermal performance of a miniature thermosyphon. Int J Heat Mass Transf.2010;53(9-10):1914-1920.
[14] GOENKA S, SANT V, SANT S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release.2014;173:75-88.
[15] CHA CY, SHIN SR, ANNABI N, et al. Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering.Acs Nano. 2013; 7(4):2891-2897.
[16] KINLOCH IA, SUHR J, LOU J, et al. Composites with carbon nanotubes and graphene: An outlook. Science.2018;362(6414):547-553.
[17] SHIMIZU M, KOBAYASHI Y, MIZOGUCHI T, et al. Carbon Nanotubes Induce Bone Calcification by Bidirectional Interaction with Osteoblasts. Adv Mater.2012;24(16):2176-2185.
[18] ZHANG L, RAKOTONDRADANY F, MYLES AJ, et al. Arginine-glycine-aspartic acid modified rosette nanotube-hydrogel composites for bone tissue engineering.Biomaterials. 2009;30(7): 1309-1320.
[19] CHUN AL, MORALEZ JG, WEBSTER TJ, et al. Helical rosette nanotubes: A biomimetic coating for orthopedics? Biomaterials. 2005; 26(35):7304-7309.
[20] ZHANG LJ, CHEN YP, RODRIGUEZ J, et al. Biomimetic helical rosette nanotubes and nanocrystalline hydroxyapatite coatings on titanium for improving orthopedic implants.Int J Nanomedicine.2008;3(3):323-333.
[21] CUI H, YU Y, LI X, et al. Direct 3D printing of a tough hydrogel incorporated with carbon nanotubes for bone regeneration.J Mater Chem B.2019;7(45):7207-7217.
[22] HEO DN, KO WK, BAE MS, et al. Enhanced bone regeneration with a gold nanoparticle-hydrogel complex.J Mater Chem B. 2014;2(11): 1584-1593.
[23] SAHA S, PAL A, KUNDU S, et al. Photochemical Green Synthesis of Calcium-Alginate-Stabilized Ag and Au Nanoparticles and Their Catalytic Application to 4-Nitrophenol Reduction. Langmuir. 2010; 26(4):2885-2893.
[24] IBANEZ JP, UMETSU Y. Uptake of trivalent chromium from aqueous solutions using protonated dry alginate beads.Hydrometallurgy. 2004; 72(3-4):327-334.
[25] JAISWAL MK, XAVIER JR, CARROW JK, et al. Mechanically Stiff Nanocomposite Hydrogels at Ultralow Nanoparticle Content. Acs Nano. 2016;10(1):246-256.
[26] CHEN ZM, KUCKLING D, TIEMANN M. Porous Aluminum Oxide and Magnesium Oxide Films Using Organic Hydrogels as Structure Matrices.Nanomaterials.2018;8(4):1-10.
[27] ELKHOURY K, RUSSELL CS, SANCHEZ-GONZALEZ L, et al. Soft-Nanoparticle Functionalization of Natural Hydrogels for Tissue Engineering Applications. Adv Healthc Mater.2019;8(18):1-14.
[28] ALLEN TM, CULLIS PR. Drug delivery systems: Entering the mainstream. Science.2004;303(5665):1818-1822.
[29] ALLEN TM, CULLIS PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev.2013;65(1):36-48.
[30] KULKARNI M, GREISER U, O'BRIEN T, et al. Liposomal gene delivery mediated by tissue-engineered scaffolds.Trends Biotechnol.2010; 28(1):28-36.
[31] MONTEIRO N, MARTINS A, REIS RL, et al. Liposomes in tissue engineering and regenerative medicine.J R Soc Interface. 2014; 11(101):1-24.
[32] KOJIMA C, KONO K, MARUYAMA K, et al. Synthesis of polyamidoamine dendrimers having poly (ethylene glycol) grafts and their ability to encapsulate anticancer drugs.Bioconjug Chem.2000; 11(6):910-917.
[33] WATHIER M, JUNG PJ, CAMAHAN MA, et al. Dendritic macromers as in situ polymerizing biomaterials for securing cataract incisions.J Am Chem Soc.2004;126(40):12744-12745.
[34] VELAZQUEZ AJ, CARNAHAN MA, KRISTINSSON J, et al. New dendritic adhesives for sutureless ophthalmic surgical procedures - In vityo studies of corneal laceration repair.Arch Ophthal.2004;122(6):867-870.
[35] JONES MC, LEROUX JC. Polymeric micelles - a new generation of colloidal drug carriers. Eur J Pharm Biopharm.1999;48(2):101-111.
[36] CHACKO RT, VENTURA J, ZHUANG JM, et al. Polymer nanogels: A versatile nanoscopic drug delivery platform.Adv Drug Deliv Rev. 2012; 64(9):836-851.
[37] 崔宇韬,刘贺,冀璇,等.智能响应性水凝胶作为药物递送系统的研究与应用[J].中国组织工程研究,2019,23(34):5508-5515.
[38] CULVER HR, STEICHEN SD, PEPPAS NA. A Closer Look at the Impact of Molecular Imprinting on Adsorption Capacity and Selectivity for Protein Templates.Biomacromolecules.2016;17(12): 4045-4053.
[39] NEVES MI, WECHSLER ME, GOMES ME, et al. Molecularly Imprinted Intelligent Scaffolds for Tissue Engineering Applications.Tissue Eng Part B Rev.2017;23(1):27-43.
[40] ZHANG Q, CHEN XH, GENG SN, et al. Nanogel-based scaffolds fabricated for bone regeneration with mesoporous bioactive glass and strontium: In vitro and in vivo characterization. J Biomed Mater Res A. 2017;105(4):1175-1183.
[41] RAHALI K, BEN MESSAOUD G, KAHN CJF, et al. Synthesis and Characterization of Nanofunctionalized Gelatin Methacrylate Hydrogels.Int J Mol Sci.2017;18(12):1-15.
[42] XIAO LX, LIU C, ZHU JH, et al. Hybrid, elastomeric hydrogels crosslinked by multifunctional block copolymer micelles.Soft Matter. 2010;6(21):5293-5297.
[43] FUJIOKA-KOBAYASHI M, OTA MS, SHIMODA A, et al. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering.Biomaterials.2012;33(30): 7613-7620.
[44] SAMADIKUCHAKSARAEI A, GHOLIPOURMALEKABADI M, EZADYAR EE, et al. Fabrication and in vivo evaluation of an osteoblast- conditioned nano-hydroxyapatite/gelatin composite scaffold for bone tissue regeneration.J Biomed Mater Res A. 2016;104(8): 2001-2010.
[45] DOROZHKIN SV. Calcium orthophosphates.J Mater Sci. 2007;42(4): 1061-1095.
[46] JARCHO M. Calcium-phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res.1981;(157):259-278.
[47] BOHNER M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury.2000;31:S37-S47.
[48] KANNAN S, GOETZ-NEUNHOEFFER F, NEUBAUER J, et al. Ionic substitutions in biphasic hydroxyapatite and beta-tricalcium phosphate mixtures: Structural analysis by rietveld refinement.J Am Ceram Soc. 2008;91(1):1-12.
[49] IIZUMI T, YAMANISHI S, KUMAGAI Y, et al. Augmentation of Helicobacter pylori urease activity by its specific IgG antibody: implications for bacterial colonization enhancement.Biomed Res.2005;26(1):35-42.
[50] STAIGER MP, PIETAK AM, HUADMAI J, et al. Magnesium and its alloys as orthopedic biomaterials: A review.Biomaterials.2006;27(9):1728-1734.
[51] MARIE PJ, AMMANN P, BOIVIN G, et al. Mechanisms of action and therapeutic potential of strontium in bone.Calcif Tissue Int. 2001;69(3): 121-129.
[52] ODELL BL. Zinc plays both structural and catalytic roles in metalloproteins. Nutr Rev.1992;50(2): 48-50.
[53] HOHLING HJ, MISHIMA H, KOZAWA Y, et al. Microprobe analyses of the potassium-calcium distribution relationship in predentine.Scanning Microsc.1991;5(1):247-255.
[54] GINTY F, FLYNN A, CASHMAN KD. The effect of dietary sodium intake on biochemical markers of bone metabolism in young women.Br J Nutr.1998;79(4):343-350.
[55] KOSE N, OTUZBIR A, PEKSEN C, et al. A Silver Ion-doped Calcium Phosphate-based Ceramic Nanopowder-coated Prosthesis Increased Infection Resistance.Clin Orthop Relat Res.2013;471(8): 2532-2539.
[56] TORRES PMC, VIEIRA SI, CERQUEIRA AR, et al. Effects of Mn-doping on the structure and biological properties of beta-tricalcium phosphate. J Inorg Biochem.2014;136:57-66.
[57] TOMOAIA G, MOCANU A, VIDA-SIMITI I, et al. Silicon effect on the composition and structure of nanocalcium phosphates In vitro biocompatibility to human osteoblasts.Mater Sci Eng C Mater Biol Appl. 2014;37:37-47.
[58] 赵呈智,张悠,付睿捷,等.骨组织工程中纳米羟基磷灰石复合材料现状、评价及展望[J].全科口腔医学电子杂志,2018,5(17):4-5.
[59] FU SZ, NI PY, WANG BY, et al. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials.2012;33(19):4801-4809.
[60] BUENO VB, BENTINI R, CATALANI LH, et al. Synthesis and characterization of xanthan-hydroxyapatite nanocomposites for cellular uptake. Mater Sci Eng C Mater Biol Appl.2014;37:195-203.
[61] WU T, TAN L, CHENG N, et al. PNIPAAM modified mesoporous hydroxyapatite for sustained osteogenic drug release and promoting cell attachment.Mater Sci Eng C Mater Biol Appl.2016;62:888-896.
[62] ROOPAVATH UK,SONI R,MAHANTA U,et al.3D printable SiO2 nanoparticle ink for patient specific bone regeneration.RSC Adv. 2019;9(41):23832-23842.
[63] XIN TW, GU Y, CHENG RY, et al. Inorganic Strengthened Hydrogel Membrane as Regenerative Periosteum.ACS Appl Mater Interfaces. 2017;9(47):41168-41180.
[64] MOUSA M, EVANS ND, OREFFO ROC, et al. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity.Biomaterials.2018;159:204-214.
[65] CUI W, LIU QQ, YANG L, et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater Sci Eng.2018;4(1):211-221.
[66] MEKA SRK, AGARWAL V, CHATTERJEE K . In situ preparation of multicomponent polymer composite nanofibrous scaffolds with enhanced osteogenic and angiogenic activities. Mater Sci Eng C Mater Biol Appl.2019;94:565-579.
[67] SURYA R, MULLASSERY MD, FERNANDEZ NB, et al. Synthesis and characterization of a clay-alginate nanocomposite for the controlled release of 5-Flurouracil.J Sci Adv Mater Devices.2019;4(3):432-441.
[68] KERATIVITAYANAN P, TATULLO M, KHARITON M, et al. Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering.ACS Biomater Sci Eng.2017;3(4):590-600.
[69] 李加乐,谭云飞,谭绪林,等.丝素蛋白水凝胶材料在组织工程中的应用研究进展[J].材料导报,2018,32(S2):176-182. |
| [1] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [2] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| [3] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [4] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Intravenous, topical tranexamic acid alone or their combination in total knee arthroplasty: a meta-analysis of randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 948-956. |
| [5] | Zhan Fangbiao, Cheng Jun, Zou Xinsen, Long Jie, Xie Lizhong, Deng Qianrong. Intraoperative intravenous application of tranexamic acid reduces perioperative bleeding in multilevel posterior spinal surgery: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 977-984. |
| [6] | Yang Xin, Jin Zhe, Feng Xu, Lu Bing. The current situation of knowledge and attitudes towards organ, eye tissue, body donation of residents in Shenyang [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 779-784. |
| [7] | Liu Bo, Chen Xianghe, Yang Kang, Yu Huilin, Lu Pengcheng. Mechanism of DNA methylation in exercise intervention for osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 791-797. |
| [8] | Zhang Guomei, Zhu Jun, Hu Yang, Jiao Hongwei. Stress of three-dimensional finite element models of E-MAX porcelain inlay [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 537-541. |
| [9] | Cheng Jun, Tan Jun, Zhao Yun, Cheng Fangdong, Shi Guojia. Effect of thrombin concentration on the prevention of postoperative cerebrospinal leakage by fibrin glue [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 570-575. |
| [10] | Li Li, Ma Li. Immobilization of lactase on magnetic chitosan microspheres and its effect on enzymatic properties [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 576-581. |
| [11] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [12] | Ye Haimin, Ding Linghua, Kong Weihao, Huang Zutai, Xiong Long. Role and mechanism of hierarchical microchanneled bone scaffolds in promoting osteogenesis and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 621-625. |
| [13] | Yu Langbo, Qing Mingsong, Zhao Chuntao, Peng Jiachen. Hot issues in clinical application of dynamic contrast-enhanced magnetic resonance imaging in orthopedics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 449-455. |
| [14] | Li Yanle, Yue Xiaohua, Wang Pei, Nie Weizhi, Zhang Junwei, Tan Yonghai, Jiang Hongjiang. Intramedullary nail fixation versus plate fixation in the treatment of displaced midshaft clavicular fractures in adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 471-476. |
| [15] | Wang Xinting, Xu Dandi, Zhang Junxia, Su Hailong Wang Qi. Stability of load-bearing cross barrier of different arch structures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3838-3843. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||