Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (26): 4249-4254.doi: 10.3969/j.issn.2095-4344.1368
Previous Articles Next Articles
Biological cage in intervertebral bone integration: histocytological properties of interface and healing mechanism of osseointegration
- Department of Limb Orthopedics and Reconstruction, Tianjin Hospital, Tianjin 300211, China
-
Received:2019-03-26 -
Contact:Zhang Tao, Associate chief physician, Department of Limb Orthopedics and Reconstruction, Tianjin Hospital, Tianjin 300211, China -
About author:Jia Peng, Attending physician, Department of Limb Orthopedics and Reconstruction, Tianjin Hospital, Tianjin 300211, China
CLC Number:
Cite this article
Jia Peng, Zhang Tao. Biological cage in intervertebral bone integration: histocytological properties of interface and healing mechanism of osseointegration[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(26): 4249-4254.
share this article
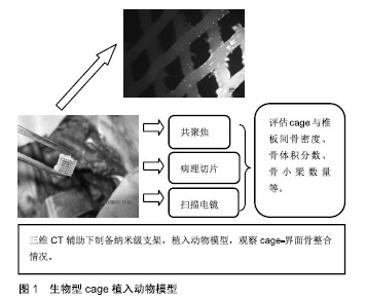
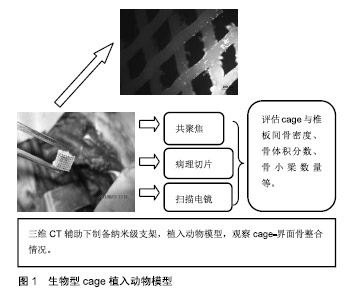
2.1 生物型cage的设计理念 Cage的设计理念、制备工艺与所选材料的种类、理化性征等因素相关。随着传统金属材料或非可吸收材料cage的弊端逐步被证实,单一可吸收材料不能满足种植体-骨界面生物力学及细胞生物学的要求,以双相或多相材料组合模式逐步替代了单相材料,成为制备cage的主流,并发挥着重要作用。 双相材料组合的必要性:传统的冷结合或热结合技术制备的单一属性cage,例如聚已内酯cage、聚乳酸 cage、聚乳酸-羟基乙酸cage等促骨再生能力弱,通常不能达到理想的融合效果。聚已内酯可以在各种环境保持其力学、物理和化学特性[3],但单纯聚已内酯植入物降解过程缓慢[4],并不能作为骨修复的理想材料。磷酸三钙不仅能弥补聚已内酯的缺陷,促进羟基磷灰石在其表面形成,为生物整合提供基础,并且能促进细胞黏附,从而诱导宿主骨-磷酸三钙界面整合[5]。Lohfeld等[6]用选择性激光烧结技术将聚已内酯与β-磷酸三钙组合,研究证明,该复合物支架可有效促进成骨细胞黏附与新骨生长,其硬度明显增强。这种双相材料组合可有效避免单一属性材料的缺陷,互相取长补短,此理念的推广,是生物型cage的发展史上的重大突破。 多相材料组合的趋势:为了优化cage力学特性及生物相容性,Ergun等[7]以聚已内酯为核心,将羟基磷灰石、磷酸三钙与聚已内酯混合制成三相cage外壳,磷酸三钙-羟基磷灰石-聚已内酯cage拥有内在相通孔道,羟基磷灰石、磷酸三钙的参与能增加材料弹性模量与骨传导性,改善力学特性;检测显示该cage具备良好的生物相容性及力学特征,符合不同力学及生物学特性的需求。基于此设计理念,可制备组合不同属性的聚合物cage。 组织工程技术的渗透:随着干细胞移植、组织工程学为特色治疗理念的不断渗透与延伸,将生物型cage作为成骨种子细胞或生长因子的负载支架,发挥组织工程化骨的生物学效应成为当今研究的热点,见图1。Wang等[8]将50 μg携带重组人骨形态发生蛋白2的肝素化聚电解质复合型cage植入猪L3-6椎间隙。结果显示融合节段新骨形成明显,骨小梁数量增多,且力学稳定。该研究证明该cage能有效地携载成骨因子,促进骨诱导,并强化骨整合。Dong等[9]脂肪干细胞和血管束填充于含有β-磷酸三钙的钛金属cage中,建立血管化骨移植模型,将富血小板血浆用于实验组。结果显示:富血小板血浆组血管生成较对照组明显,并且骨形成较对照组优越。富血小板血浆应用可有效促进血管及组织工程化骨形成。 材料表面修饰的探索:为了优化复合物cage的理化性征,强化其力学稳定性及生物相容性,对cage的表面修饰成为仿生策略发展的关键环节。材料表面修饰的手段主要有表面涂层技术、等离子体及激光表面改性技术等。Ma等[10]应用硬脂酸对β-磷酸三钙/左旋聚乳酸复合材料进行表面改性,结果发现硬脂酸与β-磷酸三钙颗粒发生反应形成羟基,经表面改性后β-磷酸三钙颗粒分布更为均匀,并且力学测试表明此种表面改性方法显着提高了β-磷酸三钙/左旋聚乳酸的机械强度。郭洪刚等[11]采用纳米表面工程技术将RGD多肽固定在经Nd∶YAG激光处理后的β-磷酸三钙/硫酸钙/聚已内酯(β-磷酸三钙/壳聚糖/聚已内酯)cage之纳米沟槽面,并对其进行生物学处理。结果显示:经这种联合改性的生物型cage具备稳定的降解特性,并且能达到椎间cage的力学要求;将成骨化脂肪干细胞接种到上述支架上,细胞生长良好。这提示经物理与化学途径修饰的表面改性支架可作为今后制备椎间cage的理想方向之一。 复合材料cage是目前研究的热点,因为某些材料如聚乳酸虽具备良好的机械性能[12],但其低骨诱导性阻碍其效果。因此,聚乳酸通常与其他材料一起使用,例如陶瓷或生物活性玻璃等,并且经表面改性后可使其更加仿生,从而提高其增强骨再生的能力[13]。随着3D打印技术的兴起,复合型cage联合表面改性以及组织工程修饰为脊柱融合提供了广阔的空间。目前已有学者基于拓扑结构进行了生物型cage动静态下的疲劳性能研究[14]。作为一种新兴技术,生物型cage的研究及应用尚处于初级阶段,需要进一步发展及探索。 2.2 动物模型 常规动物模型:Zhou等[15]将可降解多元聚氨基酸共聚物/磷酸三钙(multi-amino acidco polymer/ tri-calcium phosphate,MAACP/TCP) cage和钛金属 cage模型植入山羊C3-4椎间隙,采用非破坏刚度法对其屈、伸、旋转、侧屈方向的运动范围进行测试,结果显示:两组cage在屈、伸、侧屈方向运动范围和相对刚度无明显差异,但在旋转时上述两项MAACP/TCPcage均比钛金属cage大。Farrokhi等[16]将丙烯酸cage植入犬的颈椎,术后定量CT扫描显示:骨矿化密度、椎间隙高度均较骨移植组高,组织学分析显示cage内部可见新生骨组织及透明软骨,该实验表明丙烯酸cage具有良好的力学强度及细胞相容性。 大动物模型:Abbash等[17]将装有0.2 mg人重组骨形态发生蛋白2的医用聚已内酯-磷酸三钙cage经前路植入猪的腰椎节段,以自体骨作为对照,干预6个月时,医用聚已内酯-磷酸三钙cage完成骨整合并实现骨塑型,而在自体骨移植组25%的融合节段出现了骨断裂、吸收及假关节形成,这表明医用聚已内酯-磷酸三钙cage可以在动力载荷节段为骨再生提供适宜的环境,是一种理想的骨替代材料。 灵长类动物模型:黄帆等[18]将可吸收材料盖帽和不可吸收材料底座组合后制成部分可吸收cage,将其和聚酰胺66cage经前路随机植入恒河猴的L3-5椎间隙,术后24周行CT和micro-CT检查,进行融合定量分析,结果显示部分可吸收组融合率及融合评分明显高于对照组。术后24周显微CT扫描显示部分可吸收组cage内部骨小梁与上下椎体骨小梁融合良好,并且部分可吸收组在骨密度、骨体积分数、骨小梁数量、分离度等方面的定量分析结果均优于聚酰胺66组。灵长类骨整合模型中,可吸收性cage具有良好的力学特性及生物相容性,可以作为骨替代材料。 小动物模型:Shiels等[19]运用负载重组人骨形态发生蛋白2的85/15β-磷酸三钙/羟基磷灰石支架进行兔单一节段双侧后外侧融合,CT扫描和组织切片显示横突附近骨融合,研究强调了85/15β-磷酸三钙/ 羟基磷灰石负载重组人骨形态发生蛋白2促进脊柱融合的潜力。Han等[20]发现负载重组人骨形态发生蛋白2的工程胶原支架能增强大鼠后外侧横突间融合,进而促进脊柱融合,并认为这是一种促进骨再生和脊柱融合潜能的传递系统。 正确评估种植体-宿主骨界面间关系不仅利于深入观察骨整合过程的细微变化,并为开辟理性的研究方法对椎间骨整合机制的探索具有重要意义。动物研究中以羊为模型居多,并且大部分实验均取得了喜人成果。然而四足动物模型并不能模拟人类脊柱的退变过程,并且该类动物与人类的脊柱生物力学不完全相同[21]。鉴于医学伦理的限制,利用灵长类动物作为实验模型的研究较少,且样本量极小。对于生物型cage植入动物模型后总体疗效的评估目前多限于影像学观察,缺乏长期生物力学及临床症状的追踪[22]。因此目前能模拟人类脊柱退变动物模型的研究仍属空白。 2.3 生物型cage力学研究 生物型cage的力学特性是介导椎间骨整合的重要因素,具有理想力学特性的生物型cage可恢复椎间盘及高度,并可保持术后椎间结构的稳定性,提高融合率,降低不良反应。 运动范围的研究:Li等[23]设计了一种非植骨聚已内酯-磷酸三钙cage,具有三角形孔状蜂巢状表面,并将其与钛金属cage分别植入绵羊模型,三维CT显示:聚已内酯-磷酸三钙 cage组骨整合率及骨表面积与体积比分别是钛金属cage组的1.3倍和2.6倍,并且具有更广泛的同源骨组织。生物力学研究显示:干预6个月时,两组cage在加载负荷模式下的屈伸、侧弯运动范围均较正常节段降低;而在干预12个月时,两组间机械稳定性相当,运动范围亦无明显差异。另外,聚已内酯-磷酸三钙cage的降解与新骨形成保持同步,这一特性为脊柱融合节段所需要的结构完整性提供了保证。 力学稳定性的探索:Lu等[24]将聚乳酸和纳米级β-磷酸三钙两种可吸收材料以90∶10的比例混合制成一种新型生物可吸收cage,他们研究认为这种新型cage可为单水平颈椎间盘切除和融合提供初始稳定性。Yin等[25]运用3D打印技术制备聚乳酸/β-磷酸三钙cage,生物力学终板匹配测试显示3D打印的聚乳酸/β-磷酸三钙 cage在与颈椎终板的相容性方面优于其他cage,表明3D打印聚乳酸/ β-磷酸三钙cage具有良好的固定稳定性。 为了实现椎间融合,椎间cage必须提供足够的机械强度来承担施加在脊柱上的生理负荷[26-27],为了实现该目标,同时避免金属cage应力遮挡和下沉等不良反应,生物型cage逐渐进入研究视野。从目前基础研究来讲生物型cage生物力学研究显示其力学特性不比金属或传统cage差。Knutsen等[14]研究了用于颈椎融合的聚已内酯cage的机械疲劳特性,发现cage的设计和几何形状对静态和疲劳性能都有一定的影响,并同时指出生物型cage结合其他辅助内固定装置时效果更佳。目前关于cage-宿主骨力学研究较少,基本停留在动物体外实验层面。因此探索生物型cage的最优化设计仍是一项挑战。 2.4 生物型cage降解研究 降解过程的力学变化:Engels等[28]发现随时间延长,聚乳酸cage出现了过早失效的情况,研究者认为这与cage材料的内在形貌发生改变有关。Smit等[29]根据Engel的研究结论,对非定形聚乳酸的力学特性和形变特性进行了研究,以不同的应变速率进行抗压测试,并通过静态应力试验确定失效时间。结果证明,左旋聚乳酸比外消旋聚乳酸有更高的抗压强度,短时间内高加载速率下显示良好的强度,但是这种强度并不能长时间维持。另外,Sontjens等[30]发现非定形聚乳酸失效时间与分子量大小有关。因此cage的形貌变化与力学载荷的改变有关。 降解过程中的形貌演变:滕海军[31]等采用电镜扫描方法对取自山羊腰椎节段的外消旋聚乳酸cage切片进行观察,发现随植入时间延长,cage表面逐渐出现微裂隙、孔洞,进而分解为颗粒、泥沙样碎片,最后被新生骨替代。这表明外消旋聚乳酸在椎间植骨融合的降解过程是不等速的,早期降解主要集中在材料表面,而在后期内部降解加快。 降解过程中磨损颗粒:植入物磨损颗粒的产生与局部应力集中、降解产物刺激、材料表面特性有关[32-33]。Kienle等[34]发现钛涂层的聚醚醚酮cage在植入后出现了大小不等的磨损颗粒,并且这些磨损颗粒大部分可被吞噬细胞吞噬。Jiya等[35]也指出融合过程中骨溶解可能与cage降解产物没有直接关联,但是骨溶解却是骨不愈合或感染的重要标志,这说明椎间cage的松动、感染均可能与cage 降解过程中产生的磨损颗粒有关。 生物型cage透射线性,不会干扰术后放射成像;得益其独特的生物降解性能,生物型cage降解后在人体内不遗留任何外来物质,并且其机械性能类似松质骨,可将传统cage的应力遮挡效应降至最低[36]。然而,生物型cage的主要缺点也是由于其独特的降解特性,其与成骨效应不同步的降解速率和酸性降解产物,易诱导炎症反应、骨质溶解等不良反应,进而阻碍骨组织形成[37]。虽然生物型cage的设计理念是利用其可降解或可吸收特性更好的促进椎间融合,但是由此而导致的过早失效或力学稳定性差等弊端显著降低了生物型cage的应用效果。因此寻找最优cage参数组合在该研究领域仍是一个挑战。 2.5 生物型cage脊柱融合的临床研究 生物可吸收cage可以设计成具有与人骨相似的硬度,以减少应力遮挡,从而避免永久性植入物的风险以及二次手术的需要,不恰当植入物可能会对终板或神经结构造成损害,从而破坏融合脊柱的稳定性[38]。在骨融合过程中,避免内固定失败和cage滑移等不良反应是目前面临的主要挑战[39]。 颈椎融合:Debusscher等[40]将β-磷酸三钙-左旋聚乳酸cage植入患者退行性变颈椎,术后平均随访27个月。颈椎后凸矫正率、融合率为95%和96%,证明β-磷酸三 钙-左旋聚乳酸cage可保持椎间隙高度和良好的力学稳定性,同时为自体降解促进骨融合提供了条件。Brenke等[41]将聚乳酸-β-磷酸三钙cage植入患者颈椎,进行为期12个月的随访,结果颈痛目测类比评分、前臂疼痛目测类比评分、颈椎残障功能量表评分均明显下降,虽然其临床效果较好,但其脱出率较高。Yang等[42]研究了47例单节段颈椎前路椎间盘切除、椎间融合中羟基磷灰石/聚酰胺66 cage的临床效果,于融合状态CT扫描评估,结果证实融合率为100%,下沉率为2%,作者认为羟基磷灰石/聚酰胺66 cage可以有效促进植骨融合,防止cage下沉。 腰椎融合:Smith等[43]将70/30外消旋聚乳酸cage与碳纤维cage分别植入患者腰椎,随访1年后骨折不愈合率、螺钉崩解率、cage滑移率方面,碳纤维材料cage均优于70/30外消旋聚乳酸cage,而且碳纤维cage组的不愈合率和滑移率均为0,而70/30外消旋聚乳酸cage组中有8例发生滑移。Deng等[44]回顾性研究了羟基磷灰石/聚酰胺66和聚醚醚酮cage在腰椎间孔椎间融合术的临床效果,随访2年,结果显示两者的骨融合率分别为92.45%和91.57%,沉降率分别为7.55%和8.99%。此高融合率和低沉降率揭示了羟基磷灰石/聚酰胺66 cage力学性能可满足脊柱融合的需要。Calvo等[45]运用三维有限元分析了cage在腰椎融合过程中的主要影响因素,发现cage的尺寸、曲率及放置位置是关乎融合成败的单个主要方面,并指出cage的尺寸过宽或放置位置靠前会增加cage塌陷的风险。因此cage的几何形状对腰椎手术的成功起着至关重要的作用,cage的曲率以及放置位置应根据特定的病人确定。 综上所述,生物型cage早期可提供较好的机械强度,但长期临床效果尚无确切报道。目前,生物型cage应用于基础和临床尚处于起步阶段,但研究结果及临床追踪显示其应用意义较大。然而临床应用研究中关于生物型cage的随机对照研究很少,样本量有限,随访时间短;并且临床应用所需观察周期长,对动物模型的评估方法在临床应用有限,像显微CT和分子生物学检测等方法在人体尚不能应用;在该研究领域,对融合效果评价标准等都是亟待解决的问题。 "
| [1]Ke X, Zhang L, Yang X, et al. Low-melt bioactive glass-reinforced 3D printing akermanite porous cages with highly improved mechanical properties for lumbar spinal fusion. J Tissue Eng Regen Med. 2018; 12(5):1149-1162. [2]Li H, Yan Y, Wei J, et al. Bone substitute biomedical material of multi-(amino acid) copolymer: in vitro degradation and biocompatibility. J Mater Sci Mater Med. 2011;22(11):2555-2563. [3]Zhou Z, Yao Q, Li L, et al. Antimicrobial activity of 3D-printed poly (epsilon-Caprolactone) (PCL) composite scaffolds presenting vancomycin-loaded polylactic acid-glycolic acid (PLGA) microspheres. Med Sci Monit. 2018;24:6934-6945. [4]Daentzer D, Floerkemeier T, Bartsch I, et al. Preliminary results in anterior cervical discectomy and fusion with an experimental bioabsorbable cage-clinical and radiological findings in an ovine animal model. Springerplus. 2013;2:418. [5]Von AT, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 2006;17(4):359-366. [6]Lohfeld S, Cahill S, Barron V, et al. Fabrication, mechanical and in vivo performance of polycaprolactone/tricalcium phosphate composite scaffolds. Acta Biomater. 2012;8(9):3446-3456. [7]Ergun A, Chung R, Ward D, et al. Unitary bioresorbable cage/core bone graft substitutes for spinal arthrodesis coextruded from polycaprolactone biocomposites. Ann Biomed Eng. 2012;40(5):1073-1087. [8]Wang M, Abbah SA, Hu T, et al. Polyelectrolyte complex carrier enhances therapeutic efficiency and safety profile of bone morphogenetic protein-2 in porcine lumbar interbody fusion model. Spine (Phila Pa 1976). 2015;40(13):964-973. [9]Dong Z, Li B, Liu B, et al. Platelet-rich plasma promotes angiogenesis of prefabricated vascularized bone graft. J Oral Maxillofac Surg. 2012;70(9): 2191-2197. [10]Ma F, Chen S, Liu P, et al. Improvement of beta-TCP/PLLA biodegradable material by surface modification with stearic acid. Mater Sci Eng C Mater Biol Appl. 2016;62:407-413. [11]郭洪刚,刘静,李峰坦,等.基于三维CT图像辅助研制表面纳米化的仿生椎间融合器[J].中国组织工程研究,2012,16(21):3851-3854. [12]Llorens E, Calderon S, Del VL, et al. Polybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Mater Sci Eng C Mater Biol Appl. 2015;50:74-84. [13]Pan Z, Ding J. Poly (lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012;2(3): 366-377.[14]Knutsen AR, Borkowski SL, Ebramzadeh E, et al. Static and dynamic fatigue behavior of topology designed and conventional 3D printed bioresorbable PCL cervical interbody fusion devices. J Mech Behav Biomed Mater. 2015;49:332-342.[15]Zhou C, Song Y, Tu C, et al. Biomechanical evaluation of immediate stability of biodegradable multi-amino acid copolymer/tri-calcium phosphate composite interbody Cages in a goat cervical spine model. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011;28(1):63-66.[16]Farrokhi MR, Torabinezhad S, Ghajar KA. Pilot study of a new acrylic cage in a dog cervical spine fusion model. J Spinal Disord Tech. 2010; 23(4):272-277.[17]Abbah SA, Lam CX, Hutmacher DW, et al. Biological performance of a polycaprolactone-based scaffold used as fusion cage device in a large animal model of spinal reconstructive surgery. Biomaterials. 2009; 30(28):5086-5093.[18]黄帆.部分可吸收椎间融合器应用于恒河猴腰椎融合的研究[D].重庆:重庆医科大学,2013.[19]Shiels SM, Talley AD, McGough M, et al. Injectable and compression- resistant low-viscosity polymer/ceramic composite carriers for rhBMP-2 in a rabbit model of posterolateral fusion: a pilot study. J Orthop Surg Res. 2017;12(1):107.[20]Han X, Zhang W, Gu J, et al. Accelerated postero-lateral spinal fusion by collagen scaffolds modified with engineered collagen-binding human bone morphogenetic protein-2 in rats. PLoS One. 2014;9(5):e98480.[21]Kandziora F, Pflugmacher R, Scholz M, et al. Comparison between sheep and human cervical spines: an anatomic, radiographic, bone mineral density, and biomechanical study. Spine (Phila Pa 1976). 2001; 26(9):1028-1037.[22]丁金勇,钱莘,刘涛,等.新型组合式腰椎间融合器的研制和体内融合实验的初步研究[J].第三军医大学学报,2009,31(10):938-940. [23]Li Y, Wu ZG, Li XK, et al. A polycaprolactone-tricalcium phosphate composite scaffold as an autograft-free spinal fusion cage in a sheep model. Biomaterials. 2014;35(22):5647-5659.[24]Cao L, Duan PG, Li XL, et al. Biomechanical stability of a bioabsorbable self-retaining polylactic acid/nano-sized beta-tricalcium phosphate cervical spine interbody fusion device in single-level anterior cervical discectomy and fusion sheep models. Int J Nanomedicine. 2012;7: 5875-5880.[25]Yin X, Jiang L, Yang J, et al. Application of biodegradable 3D-printed cage for cervical diseases via anterior cervical discectomy and fusion (ACDF): an in vitro biomechanical study. Biotechnol Lett. 2017;39(9): 1433-1439.[26]Kandziora F, Pflugmacher R, Schafer J, et al. Biomechanical comparison of cervical spine interbody fusion cages. Spine (Phila Pa 1976). 2001; 26(17):1850-1857.[27]Greene DL, Crawford NR, Chamberlain RH, et al. Biomechanical comparison of cervical interbody cage versus structural bone graft. Spine J. 2003;3(4):262-269.[28]Engels TA, Sontjens SH, Smit TH, et al. Time-dependent failure of amorphous polylactides in static loading conditions. J Mater Sci Mater Med. 2010;21(1):89-97.[29]Smit TH, Engels TA, Sontjens SH, et al. Time-dependent failure in load-bearing polymers: a potential hazard in structural applications of polylactides. J Mater Sci Mater Med. 2010;21(3):871-878.[30]Sontjens SH, Engels TA, Smit TH, et al. Time-dependent failure of amorphous poly-D, L-lactide: influence of molecular weight. J Mech Behav Biomed Mater. 2012;13:69-77.[31]滕海军,周跃,范丽静,等.可吸收腰椎间融合器降解过程的动物实验研究[J].颈腰痛杂志,2010,31(1):16-19.[32]Lochner K, Fritsche A, Jonitz A, et al. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int J Mol Med. 2011;28(6):1055-1063.[33]Elsner JJ, Shemesh M, Mezape Y, et al. Long-term evaluation of a compliant cushion form acetabular bearing for hip joint replacement: a 20 million cycles wear simulation. J Orthop Res. 2011;29(12):1859-1866.[34]Kienle A, Graf N, Wilke HJ. Does impaction of titanium-coated interbody fusion cages into the disc space cause wear debris or delamination? Spine J. 2016;16(2):235-242.[35]Jiya T, Smit T, Deddens J, et al. Posterior lumbar interbody fusion using nonresorbable poly-ether-ether-ketone versus resorbable poly-L-lactide-co-D, L-lactide fusion devices: a prospective, randomized study to assess fusion and clinical outcome. Spine (Phila Pa 1976). 2009;34(3):233-237.[36]Schimmel JJ, Poeschmann MS, Horsting PP, et al. PEEK cages in lumbar fusion: mid-term clinical outcome and radiologic fusion. Clin Spine Surg. 2016;29(5):E252-E258.[37]Wuisman PI, Smit TH. Bioresorbable polymers: heading for a new generation of spinal cages. Eur Spine J. 2006;15(2):133-148.[38]Oka K, Murase T, Moritomo H, et al. Corrective osteotomy using customized hydroxyapatite implants prepared by preoperative computer simulation. Int J Med Robot. 2010;6(2):186-193.[39]Deyo RA. Fusion surgery for lumbar degenerative disc disease: still more questions than answers. Spine J. 2015;15(2):272-274.[40]Debusscher F, Aunoble S, Alsawad Y, et al. Anterior cervical fusion with a bio-resorbable composite cage (beta TCP-PLLA): clinical and radiological results from a prospective study on 20 patients. Eur Spine J. 2009;18(9):1314-1320.[41]Brenke C, Kindling S, Scharf J, et al. Short-term experience with a new absorbable composite cage (beta-tricalcium phosphate-polylactic acid) in patients after stand-alone anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2013;38(11):E635-E640.[42]Yang X, Liu L, Song Y, et al. Outcome of single level anterior cervical discectomy and fusion using nano-hydroxyapatite/polyamide-66 cage. Indian J Orthop. 2014;48(2):152-157.[43]Smith AJ, Arginteanu M, Moore F, et al. Increased incidence of cage migration and nonunion in instrumented transforaminal lumbar interbody fusion with bioabsorbable cages. J Neurosurg Spine. 2010;13(3): 388-393.[44]Deng QX, Ou YS, Zhu Y, et al. Clinical outcomes of two types of cages used in transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases: n-HA/PA66 cages versus PEEK cages. J Mater Sci Mater Med. 2016;27(6):102.[45]Calvo-Echenique A, Cegonino J, Chueca R, et al. Stand-alone lumbar cage subsidence: a biomechanical sensitivity study of cage design and placement. Comput Methods Programs Biomed. 2018;162:211-219.[46]Krijnen MR, Mullender MG, Smit TH, et al. Radiographic, histologic, and chemical evaluation of bioresorbable 70/30 poly-L-lactide-CO-D, L-lactide interbody fusion cages in a goat model. Spine (Phila Pa 1976). 2006;31(14):1559-1567.[47]Landes C, Ballon A, Ghanaati S, et al. Evaluation of the Fatigue Performance and Degradability of Resorbable PLDLLA-TMC osteofixations. Open Biomed Eng J. 2013;7:133-146.[48]Yamagata T, Takami T, Uda T, et al. Outcomes of contemporary use of rectangular titanium stand-alone cages in anterior cervical discectomy and fusion: cage subsidence and cervical alignment. J Clin Neurosci. 2012;19(12):1673-1678. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Wang Qiufei, Gu Ye, Peng Yuqin, Xue Feng, Ju Rong, Zhu Feng, Wang Yijun, Geng Dechun, Xu Yaozeng. Effect of Wnt/beta-catenin signaling pathway on osteoblasts under the action of wear particles [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3894-3901. |
| [12] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [13] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [14] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [15] | Li Xinping, Cui Qiuju, Zeng Shuguang, Ran Gaoying, Zhang Zhaoqiang, Liu Xianwen, Fang Wei, Xu Shuaimei. Effect of modification of β-tricalcium phosphate/chitosan hydrogel on growth and mineralization of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3493-3499. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||