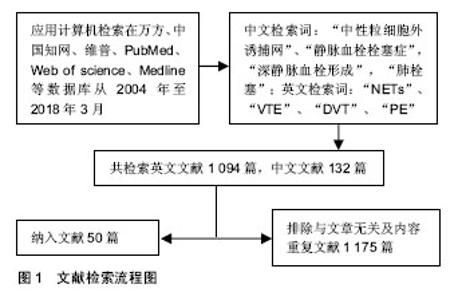
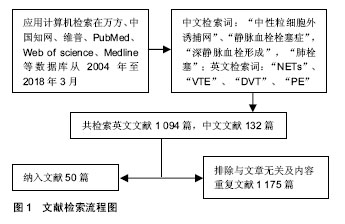
Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (12): 1930-1935.doi: 10.3969/j.issn.2095-4344.1583
Previous Articles Next Articles
Role of neutrophil extracellular traps in venous thromboembolism
Zhao Jingbin1, Zhang Xiaopeng1, Zhou Dong2
- 1Lanzhou University Second Hospital, Lanzhou 730000, Gansu Province, China; 2Department of Vascular Surgery, Lanzhou University Second Hospital, Lanzhou 730000, Gansu Province, China
-
Online:2019-04-28Published:2019-04-28 -
Contact:Zhou Dong, MD, Associate professor, Chief physician, Master’s supervisor, Department of Vascular Surgery, Lanzhou University Second Hospital, Lanzhou 730000, Gansu Province, China -
About author:Zhao Jingbin, Master candidate, Lanzhou University Second Hospital, Lanzhou 730000, Gansu Province, China
CLC Number:
Cite this article
Zhao Jingbin, Zhang Xiaopeng, Zhou Dong. Role of neutrophil extracellular traps in venous thromboembolism[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(12): 1930-1935.
share this article
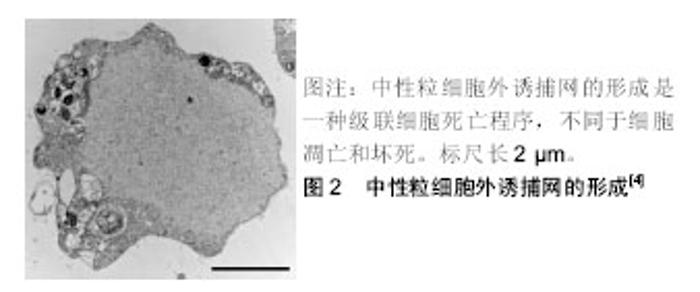
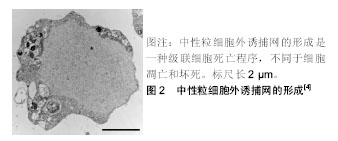
2.2 中性粒细胞外诱捕网 中性粒细胞作为机体防御的第1道防线,在机体感染的初期阶段的防御中起着重要的作用,其杀灭病原体的方式主要包括吞噬作用和脱颗粒作用。近年来发现的中性粒细胞胞外诱捕网的形成是中性粒细胞杀灭病原体的一种新的方式[3]。 中性粒细胞外诱捕网是由中性粒细胞释放到胞外的网状结构,包含有DNA、组蛋白、髓过氧化物酶、中性粒细胞弹性蛋白酶和组织蛋白酶G[4]。中性粒细胞外诱捕网的基本骨架结构是解聚的染色质,上面镶嵌着多种蛋白,包括核组蛋白、多种颗粒蛋白和一些胞浆蛋白等,这种独特的结构能形成局部的高浓度杀菌分子环 境[3,5]。实验证实,中性粒细胞外诱捕网能杀灭多种微生物,包括革兰阳性菌及阴性菌、伯氏包柔氏螺旋体、真菌和寄生虫等[6],且还可通过髓过氧化物酶和α-防御素清除人类免疫缺陷病毒[7]。 中性粒细胞外诱捕网的形成是一种级联细胞死亡程序,被称为NETosis,是一种区别于细胞凋亡和坏死的细胞死亡方式,即中性粒细胞在通过各种刺激的作用下活化,细胞核的形态发生变化,核染色质发生解聚,最后细胞膜溶解并释放出中性粒细胞外诱捕网[4],见图2;其依赖于NADPH氧化酶的活化和活性氧的产生[8]。中性粒细胞外诱捕网产生过程的主要特征为染色质发生解聚,需要组蛋白转录修饰成瓜氨酸化组蛋白,肽酰基精氨酸脱亚氨酶参与了催化过程[9],其中参与中性粒细胞外诱捕网形成的是肽酰基精氨酸脱亚氨酶4。中性粒细胞受到刺激时,肽酰基精氨酸脱亚氨酶4催化组蛋白H3、H4转化为瓜氨酸化组蛋白,使染色质发生解旋,后细胞核的形态发生改变、核膜破裂生成中性粒细胞外诱捕网[10]。中性粒细胞外诱捕网的产生与肽酰基精氨酸脱亚氨酶4的活性相关。实验表明,<10%的肽酰基精氨酸脱亚氨酶4缺陷的小鼠在下腔静脉狭窄48 h后形成了血栓,而90%的野生型小鼠下腔静脉有血栓形成,中性粒细胞大量存在于2组小鼠下腔静脉形成的血栓中,而细胞外瓜氨酸化组蛋白则仅存在于野生型小鼠下腔静脉形成的血栓中;骨髓嵌合体实验显示,造血细胞中的肽酰基精氨酸脱亚氨酶4在深静脉血栓中促进血栓形成作用的来源;通过输注野生型小鼠中性粒细胞可以活化血栓的形成,这表明中性粒细胞肽酰基精氨酸脱亚氨酶4在深静脉形成过程中是必要的[11]。"

2.3 中性粒细胞外诱捕网的来源和形成的影响因素 除相关致病微生物外,还有多种物质均能刺激中粒细胞产生中性粒细胞外诱捕网,如脂多糖、(12-)十四酸佛波酯(-13-)乙酸盐、干扰素、激活的血小板和内皮细胞以及脓毒症患者的血浆。有研究显示,含肿瘤坏死因子α、白细胞介素1β、白细胞介素8的血浆,有促进中性粒细胞产生中性粒细胞外诱捕网的作用[12]。中性粒细胞对不同刺激反应的时间是有差异的,且不同阶段的中性粒细胞对同一刺激的反应也有差异;例如,成熟中性粒细胞能在干扰素诱导及随后的补体C5a刺激下产生中性粒细胞外诱捕网,而幼稚中性粒细胞对这一刺激无反应[13]。 最初研究人员采用人早幼粒白血病HL-60细胞来进行中性粒细胞外诱捕网的体外诱导分化;后来,Marin-Esteban等[14]应用人髓样白血病细胞株PLB-985,体外诱导分化为成熟中性粒细胞后在蛋白激酶C激活剂-(12-)十四酸佛波酯(-13-)乙酸盐或过氧化氢的刺激下,将以时间和浓度依赖性的方式产生中性粒细胞外诱捕网,其成分与HL-60细胞诱导的相似;随后的研究显示,X-连锁慢性肉芽肿髓样白血病细胞株PLB-985 (X-CGD PLB-985)由于缺乏NADPH氧化酶2,在(12-)十四酸佛波酯(-13-)乙酸盐刺激时无反应,而过氧化氢可促使其产生少量中性粒细胞外诱捕网,由此证实NADPH氧化酶2和活性氧对中性粒细胞产生中性粒细胞外诱捕网起着重要的作用。 中性粒细胞胞浆内锌离子的浓度在(12-)十四酸佛波酯(-13-)乙酸盐刺激其产生中性粒细胞外诱捕网的过程中是有所变化的,应用锌离子螯合剂 N,N,N',N'-四(2-吡啶甲基)乙二胺可抑制中性粒细胞外诱捕网的产生,但不影响蛋白激酶C的激活和活性氧的产生,提示锌信号可作为活性氧依赖途径下游中的一个转导信 号[15]。此外,中性粒细胞外诱捕网的产生过程中的关键因素是染色质的解聚,此过程中需要弹性蛋白酶裂解组蛋白,使染色质变得松散而解聚[16]。缺氧诱导因子1α也能调节脂多糖诱导中性粒细胞外诱捕网的产生,其基因的沉默可使中性粒细胞外诱捕网的产生减少[17]。有研究显示,白细胞介素17A可通过ERK2途径以促进血小板的活化[18],也可通过促进血小板聚集、中性粒细胞浸润和上皮细胞的活化以促进深静脉血栓的形成[19]。 2.4 中性粒细胞外诱捕网在血栓形成过程中的作用 实验证实,在血栓形成的起始和生长阶段均有中性粒细胞外诱捕网的参与,其促进血栓形成主要是与内皮细胞、血小板、红细胞和凝血因子及其相互之间的作用有关。Fuchs等[20]发现由于中性粒细胞外诱捕网具有的独特网状结构,不仅能为血栓提供支架,且还可促进血栓的形成;在血栓形成的过程中,中性粒细胞外诱捕网不仅促使血小板黏附、聚集和激活,也为红细胞的聚集提供支架,参与招募红细胞、促进纤维蛋白沉积的过程,而诱导红色血栓的形成;当采用脱氧核糖核酸酶处理中性粒细胞外诱捕网后,其降解网状结构后阻止了血栓的形成。 2.4.1 中性粒细胞外诱捕网与内皮细胞相互作用 在血栓形成的起始阶段起着重要作用的是内皮细胞的活化和Weibel-Palade小体的释放。Pereira等[21]发现将活化的内皮细胞与中性粒细胞共同培养3 h后,可诱导产生中性粒细胞外诱捕网,并证实该过程主要依赖于内皮细胞释放的白细胞介素8。将中性粒细胞外诱捕网和内皮细胞共同培养后发现,会有大量的内皮细发生损伤,该过程在加入了NADPH氧化酶途径抑制剂二苯基碘后会消失,这表明中性粒细胞外诱捕网中的活性氧可以通过内皮细胞的损伤而促进血栓的形成[22]。Brill等[23]在小鼠体内注射纯化的组蛋白,发现其血浆内血管性血友病因子的水平升高和血栓的程度加重。有研究发现,中性粒细胞外诱捕网中的基质金属蛋白酶9通过活化内皮细胞中的基质金属蛋白酶2诱导内皮细胞损伤,从而促进血栓的形成[24]。 2.4.2 中性粒细胞外诱捕网与血小板相互作用 中性粒细胞外诱捕网作为血小板黏附聚集的支架[20]。Fuchs等[20]在体外采用悬浮血浆的血小板进行灌注中性粒细胞外诱捕网,在电子显微镜下可以观察到血小板吸附于中性粒细胞外诱捕网,后采用全血进行灌注中性粒细胞外诱捕网,可以观察到中性粒细胞外诱捕网可作为支架吸附血小板聚集。Fuchs等[20]对作为血栓支架的中性粒细胞外诱捕网和纤维蛋白进一步进行溶栓敏感性的对比研究,实验样本采用了脱氧核糖核酸酶降解中性粒细胞外诱捕网和用组织纤溶酶原激活物去除纤维蛋白,将中性粒细胞用血小板激活因子预处理并释放中性粒细胞外诱捕网,并与复钙血液进行孵育,在实验中发现,组织纤溶酶原激活物仅可去除纤维蛋白,不能阻止血栓的形成,而采用组织纤溶酶原激活物和脱氧核糖核酸酶共同处理后,当有活化的中性粒细胞存在时无血栓形成,由此可证实中性粒细胞外诱捕网作为血栓的支架不依赖纤维蛋白。 组蛋白在中性粒细胞外诱捕网促进血小板黏附和聚集中发挥着重要作用。有研究发现细胞外组蛋白对体外内皮细胞具有毒性,并可杀死大鼠,在体内,导致中性粒细胞集落、空泡化内皮细胞、肺泡内出血和大血管及微血管血栓形成[25]。Ammollo等[26]证实了细胞外组蛋白H3、H4可通过降低血栓调节蛋白依赖蛋白质C的激活作用增加血浆中凝血酶的生成。Fuchs等[20]证实了组蛋白H3、H4与血小板共同培养后有促进血小板聚集的作用,而组蛋白H1、H2A和H2B被证实无此作用。在体外,纯化组蛋白与血小板表面相结合,可能与磷脂或碳水化合物之间的静电作用,以及与血小板Toll样受体(Toll-like receptors,TLRs)相结合有关[27]。TLRs家族识别病原体相关分子模式[28],参与机体的先天免疫应答。人体内共有11种TLRs家族成员,血小板可表达其中的TLR1,TLR2,TLR4,TLR6,TLR8和TLR9[29]。不同来源的病原体相关分子模式可被不同的TLRs识别,如革兰阴性菌细胞壁脂多糖可被TLR4识别,革兰阳性菌成分可被TLR2识别,并与TLR1、TLR6共同识别细菌脂蛋白;当病原体相关分子模式与TLRs相结合后,通过细胞内复杂的信号转导途径激活核转录因子κB,激活下游相应的免疫系统,如TLR2激动剂PAM3-CSK4可诱导血小板粘连、脱颗粒、聚集和形成血小板-中性粒细胞聚集体[30]。脂多糖作为TLR4的激动剂,可诱导血小板释放ATP和表达P选择素,并且可增强通过由低浓度血小板激动剂诱导的血小板的活化[31]。联系炎症与凝血的枢纽之一可能是血小板TLRs。最近发现组蛋白可作为TLR的激动剂,Xu等[32]发现TLR2和TLR4是细胞外组蛋白介导的无菌性炎症、组织损伤和小鼠模型死亡的主要受体。Semeraro等[28]证实组蛋白在富血小板血浆中在无其他刺激因子影响的条件下,凝血酶以剂量依赖的方式进行增长,当采用单克隆抗体阻断了TLR2和TLR4时,富血小板血浆中激活的血小板下降,凝血酶的生成也随之下降,表明组蛋白在激活凝血途径时依赖TLR2和TLR4。 血小板致密颗粒中含有无机聚磷酸盐[33]。Smith等[34]在进行血浆检验分析中发现无机聚磷酸盐可激活凝血途径和激活凝血因子V参与凝血过程。Semeraro等[28]证明组蛋白在含有大豆胰蛋白酶抑制剂的贫血小板血浆中,有着增强无机聚磷酸盐促进凝血的作用。 有研究指出,高迁移率族蛋白B1促进血栓形成并促进血小板聚集,并且深静脉血栓中的高迁移率族蛋白1来自于血小板和中性粒细胞,促进血栓中的中性粒细胞外诱捕网的形成[35]。也有人指出,高迁移率族蛋白1从血小板释放增强中性粒细胞募集并促进调节血栓中中性粒细胞外诱捕网的形成,抑制NETosis过程使得血小板-高迁移率族蛋白1在急性深静脉血栓中的无促进血栓形成作用[2]。 2.4.3 中性粒细胞外诱捕网与内、外源性凝血途径 人体三大抗凝机制为抗凝血酶Ⅲ、蛋白质C和纤溶系统。中性粒细胞外诱捕网参与内源性和外源性途径的凝血过程。Kambas等[36]通过免疫印迹和激光共聚焦显微镜评估中性粒细胞外诱捕网中组织因子的表达情况,采用流式细胞仪对中性粒细胞来源的微颗粒表面组织因子进行检测,发现中性粒细胞来源的组织因子在中性粒细胞外诱捕网诱导的血栓形成过程发挥重要作用。中性粒细胞弹性蛋白酶能够裂解组织因子途径抑制剂和增强凝血因子Xa活性[37]。外源性凝血途径从释放组织因子开始,中性粒细胞外诱捕网中的弹性蛋白酶可以使组织因子途径抑制剂水解而失活,促进外源性凝血过程。von Brühl等[38]发现中性粒细胞外诱捕网可与凝血因子Ⅻ 相结合,通过内源性凝血途径促进凝血酶的生成和纤维蛋白形成。中性粒细胞外诱捕网中包含的DNA和组蛋白在促进凝血的过程中起着重要的作用,将释放中性粒细胞外诱捕网的中性粒细胞与贫血小板血浆共同培养后,血浆中凝血酶生成量增加,当加入脱氧核糖核酸酶或去除血浆中凝血因子Ⅻ和Ⅺ后,凝血酶的生成量明显下降,这表明中性粒细胞外诱捕网中DNA参与调节内源性凝血途径[39];然而,组蛋白可以活化血小板,促使血小板颗粒释放促凝血磷酸盐,表明无机聚磷酸盐在组蛋白存在的情况下,能以不依赖于因子Ⅻ方式启动凝血途 径[28,40]。有人指出,凝血因子Ⅶ活化蛋白酶可以与中性粒细胞外诱捕网的主要成分DNA和组蛋白结合并且相互作用,凝血因子Ⅶ活化蛋白酶可以比活化蛋白C更有效地降低组蛋白对内皮细胞的细胞毒性,但是,中性粒细胞外诱捕网本身没有任何促凝作用,其组分组蛋白和DNA有促凝作用[41-42]。另外,中性粒细胞外诱捕网中组蛋白与血栓调节蛋白和蛋白C相互作用,抑制血栓调节蛋白介导的蛋白C激活,从而导致血浆凝血酶生成增 加[26]。因此,中性粒细胞外诱捕网与凝血因子之间的相互作用在深静脉血栓形成过程中起着重要作用。 2.4.4 中性粒细胞外诱捕网在深静脉血栓中的作用 Brill等[23,28]分别对狒狒和小鼠深静脉血栓模型研究后发现,在深静脉血栓形成部位存在着大量中性粒细胞外诱捕网,血栓中的中性粒细胞外诱捕网可作为支架为血小板和红细胞黏附,并可促进纤维蛋白的形成,加速活化血小板和内皮细胞。有研究指出,血浆脱氧核糖核酸酶1活性降低可能导致促血栓形成中性粒细胞外诱捕网的持续存在,可促进急性血栓性微血管病患者的微血管血栓形成,中性粒细胞外诱捕网降解需要血浆中有活性的脱氧核糖核酸酶1,而重组人脱氧核糖核酸酶1可以降解血浆中的脱氧核糖核酸酶[43]。Brill等[23]报道肿瘤患者并发血栓性微血管病的血浆中DNA和中性粒细胞标记物升高水平相一致;循环细胞外DNA被广泛用作监测血栓形成中中性粒细胞外诱捕网形成的替代指标,循环细胞外DNA水平与细胞损伤标志物和缺血性损伤相关[44]。这表明中性粒细胞在血栓形成中发挥着重要作用。现在认识到,游离DNA能作为中性粒细胞外诱捕网的主要成分,由中性粒细胞主动释放。Meng等[45]通过研究多发性创伤患者血浆游离DNA/中性粒细胞外诱捕网和脱氧核糖核酸酶水平之间的相互关系后发现,发生脓毒症的患者游离DNA/中性粒细胞外诱捕网水平明显增加,在脓毒血症早期,患者血浆脱氧核糖核酸酶显著高于无脓毒症患者;脓毒症患者血浆中游离DNA/中性粒细胞外诱捕网于脱氧核糖核酸酶值无相关。 中性粒细胞外诱捕网除了对机体有消极的作用外,有研究发现,机体在产生中性粒细胞外诱捕网抵抗病原微生物的同时,多种病原体也能产生相应的抵抗机制[46],遏制中性粒细胞外诱捕网的主要机制见表1。其中研究最为广泛的属脱氧核糖核酸酶降解中性粒细胞外诱捕网的作用。分泌脱氧核糖核酸酶的A型链球菌、猪链球菌、牛支原体及疟原虫和分泌核酸内切酶A的肺炎链球菌,均水解核酸骨架结构从而降解中性粒细胞外诱捕网[47-48]。 此外,中性粒细胞外诱捕网的形成依赖于活性氧的产生,生育酚及其天然水溶性代谢物通过抑制酪氨酸磷酸化的蛋白激酶C介导的超氧阴离子的产生,可有效的抑制佛波脂对中性粒细胞的刺激作用;抗氧化剂Trolox以剂量依赖的方式清除活性氧,以抑制中性粒细胞外诱捕网的产生[49];另一种抗氧化剂Tempol既能以同样的方式清除活性氧,又可通过调节髓过氧化酶的作用影响活性氧的产生和NETosis的过程[50]。"

| [1] Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016; 149(2):315-352. [2] Dyer MR, Chen Q, Haldeman S, et al. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci Rep. 2018;8(1):2068. [3] Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663): 1532-1535. [4] Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2): 231-241. [5] Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10): e1000639. [6] Seeley EJ, Matthay MA, Wolters PJ. Inflection points in sepsis biology: from local defense to systemic organ injury. Am J Physiol Lung Cell Mol Physiol. 2012;303(5):L355-363. [7] Saitoh T, Komano J, Saitoh Y, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12(1):109-116. [8] von Köckritz-Blickwede M, Chow O, Ghochani M, et al. 7 - Visualization and Functional Evaluation of Phagocyte Extracellular Traps. Method Microbiol. 2010;37:139-160. [9] Leshner M, Wang S, Lewis C, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012 4; 3:307. [10] Brinkmann V, Laube B, Abu Abed U, et al. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp. 2010;(36): 1724. [11] Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110(21): 8674-8679. [12] Keshari RS, Jyoti A, Dubey M, et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One. 2012;7(10):e48111. [13] Martinelli S, Urosevic M, Daryadel A, et al. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem. 2004;279(42): 44123-44132. [14] Marin-Esteban V, Turbica I, Dufour G, et al. Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells. Infect Immun. 2012;80(5):1891-1899. [15] Hasan R, Rink L, Haase H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 2013;19(3):253-264. [16] Papayannopoulos V, Metzler KD, Hakkim A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677-691. [17] McInturff AM, Cody MJ, Elliott EA, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 α. Blood. 2012; 120(15): 3118-3125. [18] Zhang S, Yuan J, Yu M, et al. IL-17A facilitates platelet function through the ERK2 signaling pathway in patients with acute coronary syndrome. PLoS One. 2012;7(7):e40641. [19] Ding P, Zhang S1, Yu M1, et al. IL-17A promotes the formation of deep vein thrombosis in a mouse model. Int Immunopharmacol. 2018;57:132-138. [20] Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36): 15880-15885. [21] Pereira LF, Marco FM, Boimorto R, et al. Histones interact with anionic phospholipids with high avidity; its relevance for the binding of histone-antihistone immune complexes. Clin Exp Immunol. 1994;97(2):175-180. [22] Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2): e32366. [23] Brill A, Fuchs TA, Savchenko AS, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10(1):136-144. [24] Carmona-Rivera C, Zhao W, Yalavarthi S, et al. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2015;74(7):1417-1424. [25] Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318-1321. [26] Ammollo CT, Semeraro F, Xu J, et al. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9(9):1795-1803. [27] Blair P, Rex S, Vitseva O, et al. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009; 104(3): 346-354. [28] Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7): 1952-1961. [29] Cognasse F, Hamzeh H, Chavarin P, et al. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005; 83(2):196-198. [30] Esmon CT. Extracellular histones zap platelets. Blood. 2011; 118(13): 3456-3457. [31] Zhang G, Han J, Welch EJ, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182(12):7997-8004. [32] Xu J, Zhang X, Monestier M, et al. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187(5):2626-2631. [33] Ruiz FA, Lea CR, Oldfield E, et al. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279(43): 44250-44257. [34] Smith SA, Mutch NJ, Baskar D, et al. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006; 103(4): 903-908. [35] Stark K, Philippi V, Stockhausen S, et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood. 2016;128(20):2435-2449. [36] Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2014;73(10): 1854-1863. [37] Higuchi DA, Wun TC, Likert KM, et al. The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood. 1992;79(7): 1712-1719. [38] von Brühl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819-835. [39] Gould TJ, Vu TT, Swystun LL, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34(9):1977-1984. [40] Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143-1156. [41] Grasso S, Neumann A, Lang IM, et al. Interaction of factor VII activating protease (FSAP) with neutrophil extracellular traps (NETs). Thromb Res. 2018;161:36-42. [42] Noubouossie DF, Whelihan MF, Yu YB, et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129(8):1021-1029. [43] Jiménez-Alcázar M, Napirei M, Panda R, et al. Impaired DNase1-mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J Thromb Haemost. 2015;13(5):732-742. [44] Jiménez-Alcázar M, Kim N, Fuchs TA. Circulating Extracellular DNA: Cause or Consequence of Thrombosis? Semin Thromb Hemost. 2017;43(6):553-561. [45] Meng W, Paunel-Görgülü A, Flohé S, et al. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators Inflamm. 2012; 2012:149560. [46] Storisteanu DM, Pocock JM, Cowburn AS, et al. Evasion of Neutrophil Extracellular Traps by Respiratory Pathogens. Am J Respir Cell Mol Biol. 2017;56(4):423-431. [47] Chang Z, Jiang N, Zhang Y, et al. The TatD-like DNase of Plasmodium is a virulence factor and a potential malaria vaccine candidate. Nat Commun. 2016;7:11537. [48] Gondaira S, Higuchi H, Nishi K, et al. Mycoplasma bovis escapes bovine neutrophil extracellular traps. Vet Microbiol. 2017;199: 68-73. [49] 张凯,陈虹宇,李娇,等.中性粒细胞细胞外诱捕网的研究进展[J].中国畜牧兽医,2018,45(1):206-211.[50] Johnson CJ, Cabezas-Olcoz J, Kernien JF, et al. The Extracellular Matrix of Candida albicans Biofilms Impairs Formation of Neutrophil Extracellular Traps. PLoS Pathog. 2016;12(9): e1005884. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [3] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [4] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [5] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [6] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [7] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [8] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [9] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [10] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [11] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [12] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [13] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [14] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [15] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||