Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (12): 1936-1942.doi: 10.3969/j.issn.2095-4344.1131
Previous Articles Next Articles
Research and advances of physical therapy based on biomechanics
Jiang Yong1, Zheng Yili2, Xu Shengjia3
- 1Department of Physical Education, Jiangsu Police Institute, Nanjing 210031, Jiangsu Province, China; 2Department of Exercise and Heath, Nanjing Institute of Physical Education and Sports, Nanjing 210014, Jiangsu Province, China; 3Research Center of Military Physical Training, the PLA University of Science and Technology, Nanjing 211101, Jiangsu Province, China
-
Online:2019-04-28Published:2019-04-28 -
Contact:Xu Shengjia, Master, Assistant lecturer, Research Center of Military Physical Training, the PLA University of Science and Technology, Nanjing 211101, Jiangsu Province, China -
About author:Jiang Yong, Master, Lecturer, Department of Physical Education, Jiangsu Police Institute, Nanjing 210031, Jiangsu Province, China
CLC Number:
Cite this article
Jiang Yong, Zheng Yili, Xu Shengjia. Research and advances of physical therapy based on biomechanics[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(12): 1936-1942.
share this article
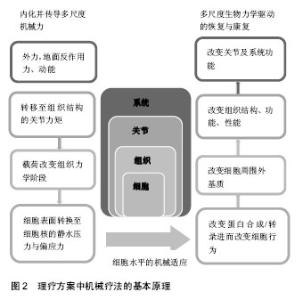
2.1 关节生物力学背景下的物理疗法 物理疗法在治疗关节疾病与骨骼肌训练方面的应用非常广泛,常采用运动疗法和操作手法的方式治疗关节疾病(如,骨关节炎、类风湿性关节炎)。运动疗法包括力量训练、关节活动度练习和有氧运动,而操作手法包括被动生理性运动和附属运动[7]。既往研究表明,这类活动有助于恢复功能、改善身体活动、减轻疼痛并增加患者满意度[1,7-8]。此外,关节松动术(如关节活动度练习、关节移动)与关节负重、力矩之间存在直接关联,而关节移动与转矩之间的相互作用又强调了物理治疗可以直接调控力对机体产生的影响。 所有的身体活动都会给机体施加一定的力和力矩。“机体为器械”,对机体施加应力与张力后,它可被看作一个机器进行整体性的移动[9-10]。从生物力学角度出发,机体的移动与位置变化取决于牛顿第二定律所描述的作用力,即力等于质量乘以加速度(F=ma)。通过机体旋转和平移的动作,使关节产生力矩,即关节力矩,是地面反作用力、关节角度与角速度共同作用的一个结果[10-11]。扩展“机体为器械”的概念,机体作为一个整体,某个关节转矩发生变化,其他关节的力向量和力矩就会进行相应的补偿与平衡[12-13]。而关节转矩作为该整体的一部分,不能将其独立,关节转矩指经过该关节的节段力量和动态耦合的总和。利用理想化的模型,可以通过关节交互结构承受的力、自由度和角速度计算力矩总和。然而,人体是一个复杂的系统,组成成分较多,并具备不同力学特性,作为一个整体,它所拥有的能力远超过现有生物力学模型所能解决的范畴。常用的生物力学模型是多刚体模型,可用于描述作用力,能够简化力学结构并明确系统的运动学特征。例如,在多刚体模型中,经过大腿和小腿的力会在膝关节处生成一个力矩,根据关节自由度和角速度,可以计算该关节力矩,它代表在膝关节处产生的节段力量和动态耦合[14-15]。 2.2 关节生物力学干预治疗的结果 2.2.1 运动疗法 已有研究将生物力学理论与临床物理治疗结果相关联[12-16]。有研究表明理疗可干预骨关节炎治疗、前交叉韧带重建和全膝关节置换,最终改变患病关节的生理学特征[11-14]。膝关节内收力矩与通过膝关节旋转中心内侧的地面反作用力的增加致膝关节内侧胫股区承受高压缩载荷,常造成膝关节炎的发生[11]。 目前已制定了运动疗法保守治疗关节疾病(包括骨关节炎)的基本原则,但无相关理论支撑,且尚未明确怎样的运动模式能够发挥最优的治疗效果,缺少对其有效性的比较,如开链运动和闭链运动[14,17]。运动疗法具有增加肌力的作用,肌力的增加又可以减轻关节负重并促使关节周围肌肉组织吸收更多的力矩,最终加速关节康复进程[17]。有研究构建了多刚体模型,阐述髋部内收肌群肌力的加强可有效减轻骨关节炎患者膝关节内侧区的载荷,研究还发现生物力学改变,如运动结合使用贴布、支架、助行器和特制鞋垫,可减轻受损或患病关节的负荷[12,14-17]。治疗骨关节炎时,步行过程中运用外翻支架与足底外侧楔形鞋垫,可使膝关节旋转中心与地面反作用力呈一直线并减少13%的膝关节内收力矩[11,13]。这些研究表明,生物力学结合运动干预可促进关节康复并限制病情恶化。 2.2.2 操作手法 操作手法可以使关节、四肢和身体其他部位发生被动运动(松动术、徒手操作术),是生物力学干预的另一种形式。松动术,指低幅振动的重复性被动运动;徒手操作术,指在关节活动范围终末端的快速牵张运动[17-18]。然而操作手法目前缺少标准化、有效的基础方案,使得其暂时不能成为一种以生物力学为基础的理疗手段。 已有研究报道,在膝关节、髋关节和盂肱关节等骨关节炎疾病的治疗过程中,操作手法能够发挥缓解疼痛、改善关节活动度与恢复生理功能的作用[7,19-21]。将操作手法、运动疗法或同时运用这两种手段与当前标准的护理措施进行对比,发现操作手法的疗效要显著优于单一的护理措施[1,7,21];而同时运用运动疗法与操作手法的手段除了增加患者满意度外,未发现其他可观察或测量的改善,这可能与治疗师的能力有关,即他对关节解剖运动(如屈、伸)和拮抗肌群(如屈肌、伸肌)牵伸知识的掌握程度[20]。由此可见,操作手法的疗效取决于治疗师所施加的运动及作用力。治疗师对生物力学关节载荷原理的理解(如关节力矩、力向量),将有利于其更好地运用运动疗法与操作手法并使其发挥治疗作用,且该原理能够推进生物力学标准方案的形成,进而维持骨骼肌健康。 在某项研究中,研究员对膝关节炎临床治疗中的关节运动参数进行了量化,包括力、频率、量(时间间隔)和振幅,结果发现治疗师自身倾向于采用相对一致的运动参数,而在不同治疗师之间却相差较多[19]。一般来说,标准化、量化的方案能够为一项技术的应用提供最佳化的指导并增强其有效性。基于此,形成一个标准化、量化的方案是该领域实现标准操作、坚持治疗、实施生物力学干预目标的第一步,同时又能够促进个性化、针对性治疗的发展。此外,也需要根据个体的关节病理、疼痛特点制定个性化的治疗方案。 2.3 关节力矩与组织和细胞水平的机械转导 身体活动(如运动疗法和操作手法产生的身体移动)可以生成与关节内部载荷直接相关的动态载荷和关节力矩[15]。步态分析与关节力矩建模显示,关节外部力矩与内部载荷之间存在一致性[12,15]。因此,关节外部力矩的“内化”和物理疗法可实现关节预期负重模式并调节关节(器官)及其构成组织的生物力学环境。关节是复杂的,是由不同性质且相互影响的组织构成的骨骼肌结构,外力一旦被“内化”,将经由不同的组织结构和附属结构来传递。需要关注在组织与细胞水平上,相关骨骼肌结构是如何传递“内化力”的。 2.4 组织水平的力传递 凭借层次结构及其功能,生物软组织具备多尺度力学特性。机体由四类不同力学性质、不同构架的组织构成,即肌肉组织、结缔组织、上皮组织、神经组织。肌肉、结缔组织作为骨骼肌系统的主要构成部分,与组织水平的机械转导密切相关,它们的力学性质取决于其组成细胞和细胞外基质成分[22-23]。2.4.1 肌腱单位 肌腱单位诱导关节转矩及其随后运动的发生,它跨过肌肉、肌腱和肌腱附着点,其层次结构使它具有独特的力学性质与传递力的功能[24]。肌肉组织对关节有支撑、稳定、运动、推进和缓冲的功能,通过肌细胞的收缩与放松产生力[25-26]。 肌筋膜实现运输肌肉力量的功能,是胶原蛋白和弹性蛋白纤维编织的连续包络膜形成的结缔组织。筋膜的层次结构:肌外膜-肌束膜-肌内膜,肌外膜包裹整个肌腹、肌束膜包绕由许多肌纤维排列构成的肌束、肌内膜包裹单条肌纤维,它们共同构成一个复杂、网状交织的胶原纤维网,使力可以连续分布。胶原纤维通过对抗拉力的方式,合成维持组织完整结构的蛋白质[22,27]。肌筋膜内卷曲的胶原纤维可用于存储能量并对器官的移动起到减震作用。在轴向拉力的作用下,胶原纤维变得“不卷曲”,延伸程度较小( < 10%)。这种情况下,胶原纤维的排列与组织所处的力学环境有关[22]。 2.4.2 骨骼 结缔组织是骨骼肌系统的一部分,通过关节节段力量和肌肉收缩对骨施加载荷,当其承受动态载荷时,压力与拉力对其产生转矩和弯矩,特别是对长骨,如股骨,此时,力由股骨头分布至股骨干,并集中于股骨颈和中段股骨干[24,28]。因为骨自身的结构,即密致骨皮质与松质骨小梁基质组织,它可以承受较大的压力与拉力。矿化的微结构与皮质骨的薄状蛋白质网络层(似洋葱圈状)构成与应力集中相关的骨皮质区域,而形成骨小梁网格组织的材质与结构,则更有助于骨的灵活性与其吸收冲击载荷的作用[29]。 既往研究表明,细胞外周流体的流动是力间接转移和骨机械转导的一个重要途径。液体填充空间沿骨分布,动态应变下,其层状网络结构为细胞外周流体的流动提供了条件。此外,流体优先受到应力改变区域的驱动,进而发生大量非线性运输并诱导亚细胞信号通路[30]。通过流体流动,并借助骨膜下区域(骨与骨膜间)转移流体的静水压,流体的方向与速率取决于骨膜自身的渗透性[31]。骨作为一个可以诱导流体流动的多孔弹性材料,协助完成机械-化学传导、相关过程的重塑/构建、力和关节水平至细胞水平机械载荷转移的调节[32]。 骨骼肌系统的层次结构与体系结构为组织水平多尺度力的传递提供了途径。结缔组织使得这些结构紧密结合,如生成骨膜并作为夏普氏纤维深入皮质骨的肌筋膜连续胶原纤维,胶原纤维组成的结缔组织能够对抗载荷与周围组织结构给予的压力[33]。由于关节组织结构相互连结,关节扭转产生的动态载荷会被分布到关节并向周围骨骼肌组织转移。 2.5 细胞水平的力传递 在细胞水平,关节节段力最终被传递至骨骼肌组织的机械敏感性细胞,此时,表现为组织膨胀应变(如静水压)和(或)作用于细胞的偏应力(如剪切力)[34]。又因为细胞位于复杂且不稳定的环境中,所以膨胀应变或偏应力不会单独存在于机体细胞上。为实现在关节治疗过程中实际应用细胞机械转导理论,细胞所处机械环境可被认为是局部环境中主要的机械力环境,这可能会受到整体的力传导的显著影响。 2.5.1 黏着斑、细胞骨架、细胞表面受体 凭借黏着斑、细胞骨架和细胞表面受体,机械敏感性细胞可感知到局部张力。黏着斑是机械敏感性蛋白结构,可抵抗细胞结构中的张力、使细胞形成机械性连结并牵引至细胞外基质[35-36]。细胞骨架则由多个坚韧且具有弹性的单纤维构成,包括肌动蛋白(拉力)和微管蛋白(压力)。研究发现,机械刺激能够改变细胞的整体形状和刚度,而细胞形状与结构又会对细胞的机械转导、下游细胞信号和分化造成巨大影响,与黏着斑对抗张力的功能相似,细胞骨架使得细胞可抵抗机械刺激引起的形变[37-38]。细胞表面受体则是反映应力诱导下的细胞膜的构象变化,通过生化过程及下游细胞信号通路,它能够调节细胞的机械敏感性(如牵拉激活通道),进而改变细胞行为和蛋白合成[39]。 2.5.2 机械敏感性细胞 机体中存在大量机械敏感性细胞,位于或途经骨骼肌组织的3类机械敏感性细胞:骨髓间充质干细胞、骨细胞和红细胞。这些细胞可用于对照实验,如既发挥传感器作用又充当机械刺激与机械转导促动器的非特化细胞、强附着性细胞(如骨细胞)和完全能动性细胞(如红细胞)各自具有特异性结构并能够将机械刺激转变为可被机体识别的生物信号[34]。 间充质干细胞的机械敏感性是成熟细胞机械敏感性(约0.02 Pa)的103倍,如骨细胞和软骨细胞(1.0- 2.0 kPa)[40]。凭借与周围细胞外基质的信息交流和自身细胞骨架,骨骼肌组织中的间充质干细胞能够感知到机械刺激。该过程由不同的细胞内信号通路调节,如激活促有丝分裂蛋白激酶通路进而改变黏着斑、细胞外基质周围黏附细胞和细胞膜的机械整合通路[39]。 骨细胞是骨骼主要的机械传感器,对流体孔隙进行填充,然后通过断裂小管与其他细胞形成连结网络,包括:骨细胞、成骨细胞、骨膜细胞以及骨髓微环境[41-42]。又因为骨细胞周围充满液体,它能够直接感知到动态水压和作用在细胞体与细胞突上的剪切力[43]。而细胞外流场可直接被矿化基质和细胞突调节,通过其限定矿化阶段与纳米/组织学相关细胞外空间的功能进行调节。流体施加在骨细胞突表面和骨细胞体的阻力,会在骨细胞上生成偏应力与膨胀应力,随时间的推移,能够调节细胞外基质的重塑/构建及矿化过程[43-44]。同时,流体可诱导骨细胞沿流动方向牵伸、应变,从而导致细胞本身的几何结构发生变化[38]。 因此,细胞能够感知到其表面导致细胞形变的偏应力和膨胀应力,其组成(黏着斑、细胞骨架和牵拉激活通路)与细胞外基质发挥感知应力及在细胞间转换形变的作用。而细胞质中的肌动蛋白、微管蛋白和中等纤维则呈现为各向异性,从而为直接调节细胞核形状和量提供了可能途径[35]。即信号通路和细胞骨架将力传导至细胞核,导致其构象改变,而这一改变又会直接或间接调控蛋白的转录。细胞机械环境的改变使得细胞外基质参与下游应力和细胞张力的调节[34]。 2.6 细胞调节与组织重塑/构建所诱导的机械适应的分子机制 细胞与组织层面的适应和重建,涉及细胞生物力学反应、组织内活性物质和机械载荷等问题。膨胀应力与偏应力除上述功能外,还参与调节细胞分化,其细胞系定型取决于身体活动产生的应力和张力的量级与方 向[36]。近期,相关配对实验与计算研究已开始着手“绘制机械基因组”[45]。此外,细胞外基质结构蛋白转录调节因子能够应答机械刺激,如胶原蛋白、弹性蛋白和蛋白聚糖,在细胞的整个生命周期中,细胞外基质的组成与结构又能够体现细胞的受力状况并记录局部环境的形成过程。 细胞与细胞外基质之间相互作用,细胞负责细胞外基质的构建与重建,细胞外基质则组成细胞的局部环境,一般由胶原蛋白和弹性蛋白纤维网构成,这类自调整的纤维网可应答细胞的力学-化学-生物环境。此外,细胞外基质能够反映细胞内部的活动,包括基因转录、蛋白合成、修饰后翻译和细胞重建(如成骨细胞沉积和破骨细胞再吸收)等,机械性地调节生化刺激[46]。细胞外基质、细胞微环境和组织结构的构建同时发生,也因此,细胞外基质的体系结构需要不断地调整以感知其机械环境和细胞自调节环境。近年来,细胞生物力学诱导的组织机械适应成为了一个新兴领域,甚至有研究表明,恰当的力学刺激能够促进靶向分子和细胞介导的组织机械适应的发生[47]。 2.7 机械疗法驱动的系统机械适应 所有的身体活动均会使机体生成机械力。除运动疗法和操作手法外,关节负重、低振幅、动态水压刺激被证实具有治疗效益,以力学干预的方式使机体进行重复性运动,进而促进组织的合成代谢能够满足治愈的需求,见图2。除此之外,体外冲击波疗法,也已用于临床,作为诱导组织愈合与重建的手段。上述所有方式均为无创手段,主要应用于骨骼肌组织,包括骨、关节和肌腱/韧带。"
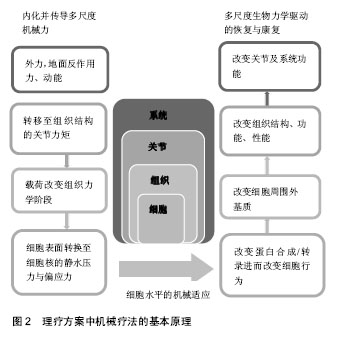

2.7.1 关节负重 关节负重是机械疗法的一种形式,常应用于理疗,包括运动疗法、操作手法和松动术。关节所受的力,即关节载荷指通过该关节的节段力与动态耦合的总负荷,其合成和分解代谢能力可用于阐述关节载荷的力学机制;关节代谢能力提高,转化四肢髓腔内压力的能力随之提高[48]。在较小的尺度上,关节载荷能够调节由应力和压力梯度驱动的分子间隙的运输能力,分子间隙内含有化学梯度和促组织重建的流动电位[48]。研究发现微小的应力,即 < 20微应力,利于手术创口愈合、促结痂形成并显著增加胫骨与股骨的皮质厚度[48,49]。因此,关节负重是一类促进愈合和组织再生的有效机械刺激方式。 2.7.2 低量级机械信号 无论是振动还是震荡生成的低量级机械信号均能提高骨组织的合成代谢能力,如骨折后,经6-12个月的低量级机械信号治疗,发现患者骨小梁和骨皮质的强度得到有效增加。采用高频低量级载荷进行相同研究,发现组织对高频活动存在生物敏感性,且该低量高频刺激使骨小梁强度增加34%[50]。骨强度的增加提示了低量级机械信号与低量高频可能诱导合成代谢,同体外冲击波疗法一样,需要更多的临床试验以证明该推测。 2.7.3 动态水压刺激 是一中无创的传输动态压力手段。对肌萎缩大鼠模型使用动态水压刺激技术,即在其胫骨周围肌肉组织交替使用30 mm Hg(1 mm Hg= 0.133 kPa)的动、静态水压载荷,与空白组相比,动态水压刺激使胫骨增加了83%骨量和190%骨附着率[51]。动态水压刺激可用于阐述关节负重促进合成代谢的机制,即施加于四肢的机械刺激可直接调控髓腔内流体的流动和压力大小,进而产生生物力学应答。 2.7.4 脉冲磁场疗法与体外冲击波疗法 外源性生物物理疗法能够促进组织自然修复,从而获得更高的治疗效益,如脉冲磁场疗法和体外冲击波疗法,普遍认为这类疗法在炎症通路调节、生长因子上调、蛋白合成中发挥作用[52-53]。近期研究发现,脉冲磁场疗法除在骨、关节损伤方面发挥作用,还可有效增加肌肉质量并促进肌腱修复。体外冲击波疗法,又被称为碎石术,广泛用于治疗运动损伤(如肌腱病变和足底筋膜炎)、骨再生障碍和所有组织的慢性伤口[54]。 体外冲击波疗法的治疗机制尚处于研究阶段,动物实验提示,该修复机制主要依靠其控制组织损伤的能力,即外源性诱导的微损伤或“磨损”,运动与身体活动时常发生此类损伤[55]。微损伤累积应答重复性载荷(又称疲劳载荷),而微损伤又是促修复的内源性诱因。尽管,脉冲磁场疗法、体外冲击波疗法可潜在加速内源性愈合,但仍需要进一步的临床试验以证实其疗效并制定最佳的治疗方案[56-57]。"

| [1] Ng JL, Kersh ME, Kilbreath S, et al. Establishing the basis for mechanobiology-based physical therapy protocols to potentiate cellular healing and tissue regeneration. Front Physiol. 2017;8:303-316. [2] Thompson WR, Scott A, Loghmani MT, et al. Understanding mechanobiology: physical therapists as a force in mechanotherapy and musculoskeletal regenerative rehabilitation. Phys Ther. 2016;96(4):560-569. [3] Knothe Tate ML, Gunning PW, Sansalone V. Emergence of form from function - mechanical engineering approaches to probe the role of stem cell mechanoadaptation in sealing cell fate. Bioarchitecture. 2016;6(5):85-103. [4] Knothe Tate ML. Top down and bottom up engineering of bone. J Biomechanics. 2011;44(2):304-312. [5] Anderson EJ, Knothe Tate ML. Design of tissue engineering scaffolds as delivery devices for mechanical and mechanically modulated signals. Tissue Eng. 2007;13(13): 2525-2538. [6] Warden SJ, Mantila Roosa SM, Kersh ME, et al. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci USA. 2014;111 (14): 5337-5342. [7] Jansen MJ, Viechtbauer W, Lenssen AF, et al. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review. J Physiother. 2011;57(1):11-20. [8] French HP, Cusack T, Brennan A, et al. Exercise and manual physiotherapy arthritis research trial (EMPART) for osteoarthritis of the hip: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2013;94(2):302-314. [9] Nicholls DA, Gibson BE. The body and physiotherapy. Physiother Theory Pract. 2010;26:497-509. . [10] Wardrope J, Barron D, Draycott S, et al. Soft tissue injuries: principles of biomechanics, physiotherapy and imaging. Emerg Med J. 2008;25(3):158-162. [11] Farrokhi S, Voycheck CA, Tashman S, et al. A biomechanical perspective on physical therapy management of knee osteoarthritis. J Orthop Sports Phys Ther. 2013;43(9): 600-619. [12] Block JA, Shakoor N. Lower limb osteoarthritis: biomechanical alterations and implications for therapy. Curr Opin Rheumatol. 2010;22(5):544-550. [13] Yaari L, Kosashvili Y, Segal G, et al. A novel non-invasive adjuvant biomechanical treatment for patients with altered rehabilitation after total knee arthroplasty: results of a pilot investigation. Clin Orthop Surg. 2015;7(2):191-198. [14] Fleming BC, Oksendahl H, Beynnon BD. Open- or closed-kinetic chain exercises after anterior cruciate ligament reconstruction? Exerc Sport Sci Rev. 2005;33(3):134. [15] Anderson DE, Madigan ML, Nussbaum MA. Maximum voluntary joint torque as a function of joint angle and angular velocity: model development and application to the lower limb. J Biomech. 2007;40(14):3105-3113. [16] Shepherd R, Carr J. Reflections on physiotherapy and the emerging science of movement rehabilitation. Aust J Physiother.1994;40S(40S):39-47. [17] Page CJ, Hinman RS, Bennell KL. Physiotherapy management of knee osteoarthritis. Int J Rheum Diss. 2011; 14(2):145-151. [18] French HP. Physiotherapy management of osteoarthritis of the hip: a survey of current practice in acute hospitals and private practice in the Republic of Ireland. Physiotherapy. 2007;93(4):253-260. [19] Tragord B S, Gill N W, Silvernail J L, et al. Joint mobilization forces and therapist reliability in subjects with knee osteoarthritis. J Man Manipulative Ther. 2013;21(4):196-206. [20] French HP, Cusack T, Brennan A, et al. Exercise and manual physiotherapy arthritis research trial (EMPART) for osteoarthritis of the hip: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2013;94(2):302-314. [21] Crowell MS. Impaired movement in a patient with glenohumeral joint osteoarthritis: a case report. J Orthop Sports Phys Ther. 2015;45(6):453-459. [22] Culav E M, Clark C H, Merrilees M J. Connective tissues: matrix composition and its relevance to physical therapy. Phys Ther. 1999;79(3):308-319. [23] Knothe Tate ML. "Whither flows the fluid in bone? " An osteocyte's perspective. J Biomech. 2003;36(10):1409-1424. [24] Prendergast PJ, Huiskes R, Søballe K. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech. 1997, 30(30):539-548. [25] Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture. 2003;17(2):159-169. [26] Hamner SR, Seth A, Delp SL. Muscle contributions to propulsion and support during running. J Biomech. 2010; 43(14):2709-2716. [27] Viguet-Carrin S, Garnero P, Delmas P D. The role of collagen in bone strength. Osteoporosis Int, 2006, 17(3):319-336. [28] Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle coordination of human walking: Part I: introduction to concepts, power transfer, dynamics and simulations. Gait Posture. 2002;17(1):215-232. [29] Rho JY, Kuhnspearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998; 20(2):92. [30] Knothe Tate ML, Steck R, Forwood MR, et al. In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Biol. 2000;203(18):2737-2745. [31] Sarah F Evans, Jonathan B Parent, Colin E Lasko, et al. Periosteum, bone's “smart” bounding membrane, exhibits direction-dependent permeability. J Bone Miner Res. 2013; 28(3):608-617. [32] Tate MLK, Yu NYC, Jalilian I, et al. Periosteum mechanobiology and mechanistic insights for regenerative medicine. Bonekey Rep. 2016;5:857. [33] Moore SR, Milz S, Tate MLK. The linea aspera: a virtual case study testing emergence of form and function. Anat Rec. 2013; 297(2):273-280. [34] Knothe Tate ML, Falls TD, Mcbride SH, et al. Mechanical modulation of osteochondroprogenitor cell fate. Int J Biochem Cell Biol. 2008;40(12):2720-2738. [35] Fouchard J, Bimbard C, Bufi N, et al. Three-dimensional cell body shape dictates the onset of traction force generation and growth of focal adhesions. Proc Natl Acad Sci USA. 2014;111 (36):13075-13080. [36] Lamothe JM, Hamilton NH, Zernicke RF. Strain rate influences periosteal adaptation in mature bone. Med Eng Phys. 2005;27(4):277-284. [37] Ingber D E. Tensegrity-based mechanosensing from macro to Micro. Prog Biophys Mol Biol. 2008;97(3):163-179. [38] Chang H, Tate M. Structure-function relationships in the stem cell’s mechanical world B: emergent anisotropy of the cytoskeleton correlates to volume and shape changing stress exposure. Mol Cell Biomech. 2011;8:297-318. [39] Rocio SM, Moroni M, Lewin GR, et al. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife. 2017;6:e21074. [40] Mcbride SH, Falls T, Knothe Tate ML. Modulation of stem cell shape and fate B: mechanical modulation of cell shape and gene expression. Tissue Eng A. 2008;14(9):1573-1580. [41] Burger EH, KleinNulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. FASEB J. 1999;13(8):S101-112. [42] Tate M L, Fath T. The only constant is change: next generation materials and medical device design for physical and mental health. Adv Healthc Mater. 2016;5(15):1840-1843. [43] Anderson EJ, Kaliyamoorthy S, Alexander JID, et al. Nano–microscale models of periosteocytic flow show differences in stresses imparted to cell body and processes. Ann Biomed Eng. 2005;33(1):52-62. [44] Anderson E J, Knothe Tate. Idealization of pericellular fluid space geometry and dimension results in a profound underprediction of nanomicroscale stresses imparted by fluid drag on osteocytes. J Giomech. 2008;41:1736-1746. [45] Song M, Brady-Kalnay S, Mcbride S, et al. Mapping the mechanome of live stem cells using a novel method to measure local strain fields in situ at the fluid-cell interface. PLoS One. 2012;7:e43601. [46] Ng J, Knothe L, Whan R, et al. Scale-up of nature’s tissue weaving algorithms to engineer advanced functional materials. Sci Rep. 2017;7:40396. [47] Huang C, Holfeld J, Schaden W, et al. Mechanotherapy: revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol Med. 2013;19(9):555-564. [48] Zhang P, Sun Q, Turner CH, et al. Knee loading accelerates bone healing in mice. J Bone Miner Res. 2007;22(12): 1979-1987. [49] Freutel M, Seitz A M, Galbusera F, et al. Medial meniscal displacement and strain in three dimensions under compressive loads: MR assessment. J Magn Reson Imaging. 2014;40(5):1181-1188. [50] Ozcivici E, Luu YK, Rubin CT, et al. Low-level vibrations retain bone marrow's osteogenic potential and augment recovery of trabecular bone during reambulation. Plos One. 2010;5(6): e11178. [51] Yokota H, Tovar A, Robling A. Dynamic muscle loading and mechanotransduction. Bone. 2012;51(4):826-827. [52] Rosso F, Bonasia DE, Marmotti A, et al. Mechanical stimulation (pulsed electromagnetic fields “pemf” and extracorporeal shock wave therapy “eswt”) and tendon regeneration: a possible alternative. Front Aging Neurosci. 2015;7:211. [53] Zhai M, Jing D, Tong S, et al. Pulsed electromagnetic fields promote in vitro osteoblastogenesis through a Wnt/β‐catenin signaling‐associated mechanism. Bioelectromagnetics. 2016;37(3):152-162. [54] Vahdatpour B, Sajadieh S, Bateni V, et al. Extracorporeal shock wave therapy in patients with plantar fasciitis. A randomized, placebo-controlled trial. J Res Med Sci. 2012;17: 834-838. [55] Knothe Tate ML, O'Leary J, Mcnamara E, et al. Lithotripsy stimulates new bone formation and mitigates loss of bone due to disuse in aged rats. Technol Health Care. 2013;21(6): 587-597. [56] Morsy EHFE, Mohamed MGS, Mahmoud EMT. Electromagnetic field versus circuit weight training on bone mineral density in elderly women. Clin Interv Aging. 2015; 10(1):539-548.[57] Hannemann PFW, Essers BAB, Schots JPM, et al. Functional outcome and cost-effectiveness of pulsed electromagnetic fields in the treatment of acute scaphoid fractures: a cost-utility analysis. BMC Musculoskelet Disord. 2015;16:84. |
| [1] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [2] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [3] | Lü Zhen, Bai Jinzhu. A prospective study on the application of staged lumbar motion chain rehabilitation based on McKenzie’s technique after lumbar percutaneous transforaminal endoscopic discectomy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1398-1403. |
| [4] | Chen Xinmin, Li Wenbiao, Xiong Kaikai, Xiong Xiaoyan, Zheng Liqin, Li Musheng, Zheng Yongze, Lin Ziling. Type A3.3 femoral intertrochanteric fracture with augmented proximal femoral nail anti-rotation in the elderly: finite element analysis of the optimal amount of bone cement [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1404-1409. |
| [5] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [6] | Chen Jinping, Li Kui, Chen Qian, Guo Haoran, Zhang Yingbo, Wei Peng. Meta-analysis of the efficacy and safety of tranexamic acid in open spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1458-1464. |
| [7] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [8] | Xu Yulin, Shen Shi, Zhuo Naiqiang, Yang Huilin, Yang Chao, Li Yang, Zhao Heng, Zhao Lu. Biomechanical comparison of three different plate fixation methods for acetabular posterior column fractures in standing and sitting positions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 826-830. |
| [9] | Cai Qunbin, Zou Xia, Hu Jiantao, Chen Xinmin, Zheng Liqin, Huang Peizhen, Lin Ziling, Jiang Ziwei. Relationship between tip-apex distance and stability of intertrochanteric femoral fractures with proximal femoral anti-rotation nail: a finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 831-836. |
| [10] | Song Chengjie, Chang Hengrui, Shi Mingxin, Meng Xianzhong. Research progress in biomechanical stability of lateral lumbar interbody fusion [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 923-928. |
| [11] | Xie Chongxin, Zhang Lei. Comparison of knee degeneration after anterior cruciate ligament reconstruction with or without remnant preservation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 735-740. |
| [12] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [13] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [14] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [15] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||