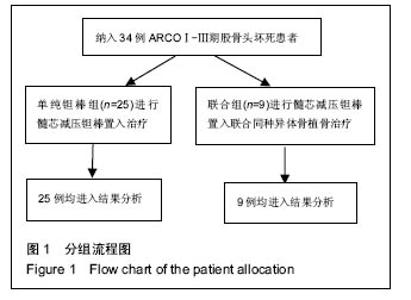
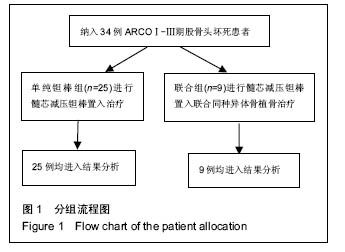
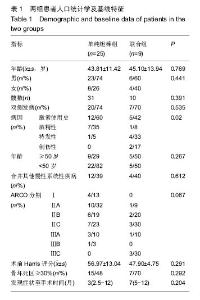
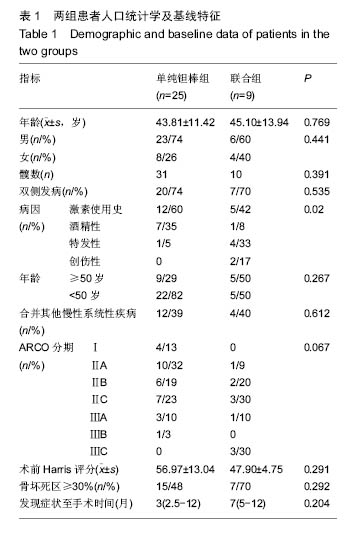
Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (47): 7043-7050.doi: 10.3969/j.issn.2095-4344.2016.47.007
Previous Articles Next Articles
Therapeutic effect of porous tantalum implantation for osteonecrosis of the femoral head and construction of a prognostic model
- Department of Orthopaedics, the Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2016-10-06Online:2016-11-18Published:2016-11-18 -
Contact:Wei Xiao-chun, Professor, Chief physician, Doctoral supervisor, Department of Orthopaedics, the Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Mao Zi-mu, Studying for master’s degree, Department of Orthopaedics, the Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
CLC Number:
Cite this article
Mao Zi-mu, Yin Kun, Wang Yu-ze, Li Peng-cui, Guo Li, Wei Xiao-chun .
share this article
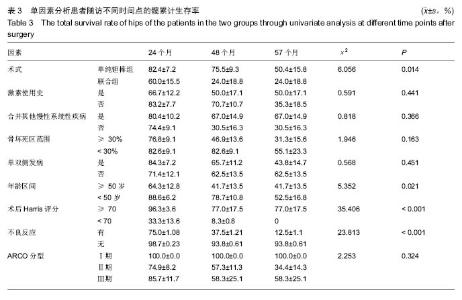
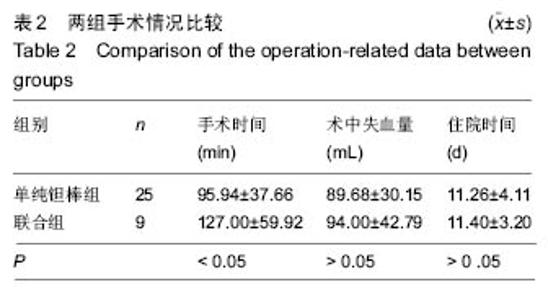
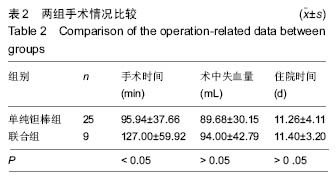
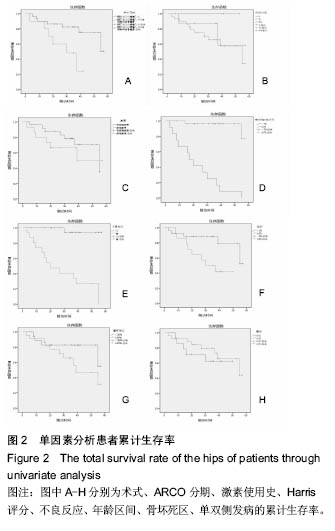
2.4 两组治疗后Harris评分变化 末次随访时,单纯钽棒组、联合组的Harris评分分别为78.52±18.37、63.30±17.42,两组Harris评分均高于治疗前(P < 0.05),且单纯钽棒组Harris评分高于联合组(P < 0.05)。 2.5 出现终点事件的髋生存分析和预后因素评估 末次随访时,单纯钽棒组31髋中,有8髋出现终点事件,其中1髋转归至全髋关节置换;联合组10髋中,有6髋出现终点事件,其中3髋转归至全髋关节置换。对两组进行Kaplan-Meier生存分析,结果示末次随访时,单纯钽棒组髋累计生存率为(50.4±15.8)%,联合组累计生存率为(24.0±18.8)%,两组髋累计生存率比较差异有显著性意义(χ2=6.056,P=0.014),见表3,图2。"
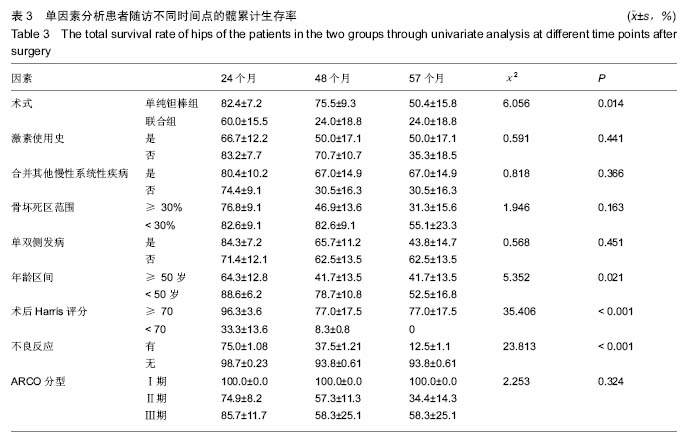
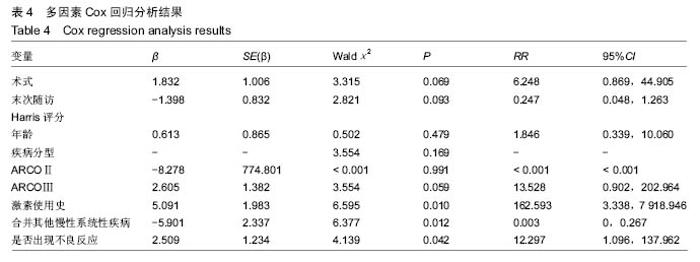
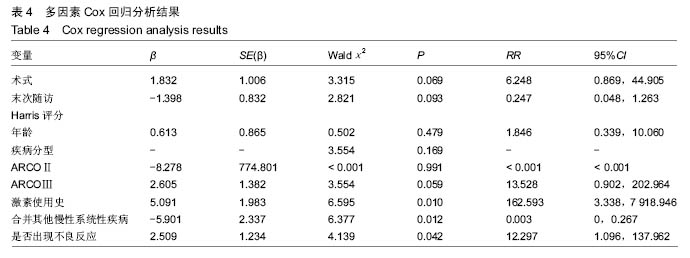
根据当前所研究的数据显示,激素使用史 (χ2=0.591,P=0.441)、是否合并其他慢性系统疾病(χ2=0.818,P=0.366)、骨坏死区范围30%分界 (χ2=1.946,P=0.163)、单双侧发病(χ2=0.568,P=0.451)、ARCO分型(χ2=2.253,P=0.324)分层研究术后生存曲线差异无统计学意义。 Cox多因素分析显示,激素使用史(RR=162.593,P=0.010)、合并其他慢性系统性疾病(RR=0.319,P=0.014)、是否出现不良反应(RR=12.297,P=0.042)为转归至出现终点事件的独立预后因素。术式、年龄、ARCO分期、末次随访Harris评分等因素与出现终点事件无统计学相关性,见表4。"
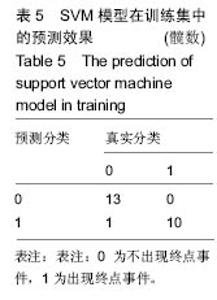
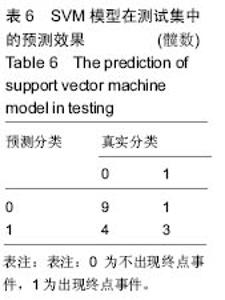
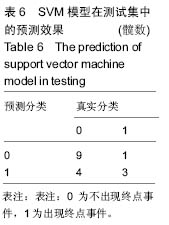
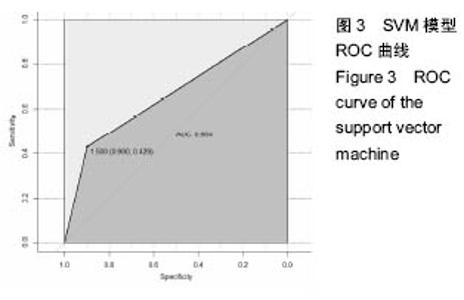
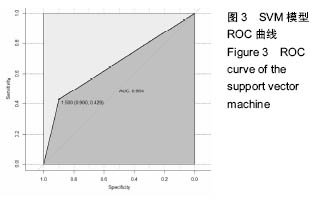
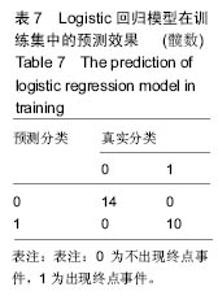
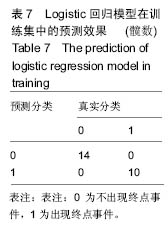
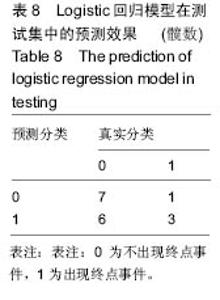
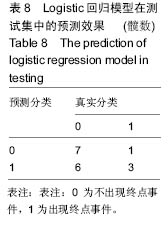
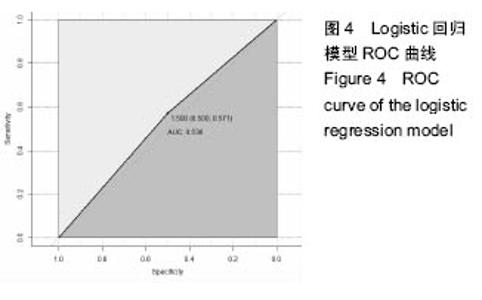
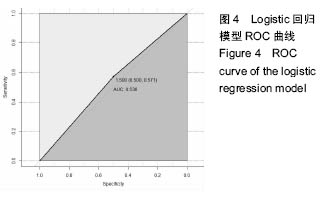
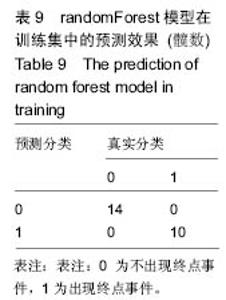
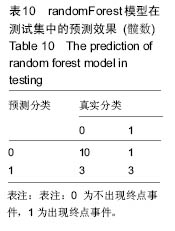
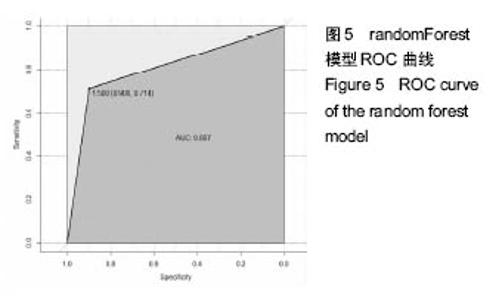
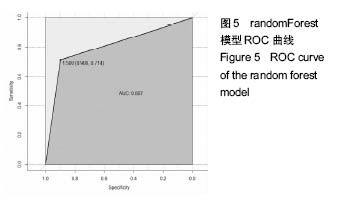
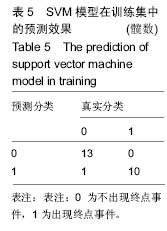
2.6 数学建模 为了更好地确定钽棒置入时机和不同术式的疗效差别,作者对所有纳入研究的病例进行了股骨头坏死钽棒置入手术预后预测模型的数学建模[9],通过提取手术前后相关数据,构造数据集进行建模及预测。数学模型选取支持向量机(support vector machine,SVM),随机森林(randomForest)两种数学模型并与Logistic回归分析进行模型对比[10],所有建模过程均在R语言下完成。建模选取19个变量,分别为性别、年龄、住院时间、手术持续时间、术中出血量、单双侧发病、术式、ARCO分型、术前Harris评分、末次随访Harris评分、发病至入院时间、随访时长、致病因素、激素使用史、是否合并其他慢性系统性疾病、实施手术髋数、骨坏死区30%分界、有无不良反应、切口愈合情况,预测变量为结果事件有无。结果显示,SVM模型在训练集中预测准确率为95.8%,在测试集中预测准确率为70.6%;randomForest模型在训练集中预测准确率为100%,在测试集中预测准确率为76.5%;Logistic回归模型在训练集中预测准确率为100%,在测试集中预测准确率为58.8%。randomForest模型预测准确率最高,在小样本条件下已显示出优越的预测性能。因此作者认为randomForest模型可较好地用于预测术后情况,对于患者术后跟进具有实际价值,可作为预测该术式5年生存期的有效方法。 2.6.1 SVM模型 SVM模型在训练集中的预测效果,在训练集中预测准确率为95.8%,训练集中真实塌陷率为41.7%,预测塌陷率为45.8%,见表5。"
| [1]Wang XS,Zhuang QY,Weng XS,et al.Etiological and clinical analysis of osteonecrosis of the femoral head in Chinese patients.Chin Med J (Engl).2013;126(2): 290-295. [2]Morse CG,Mican JM,Jones EC,et al.The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis.2007;44(5):739-748. [3]Mont MA,Cherian JJ,Sierra RJ,et al.Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? A Ten-Year Update.J Bone Joint Surg Am.2015;97(19):1604-1627. [4]Zhang Y,Li L,Shi ZJ,et al.Porous tantalum rod implant is an effective and safe choice for early-stage femoral head necrosis: a meta-analysis of clinical trials.Eur J Orthop Surg Traumatol.2013;23(2):211-217. [5]Symptomatic multifocal osteonecrosis.A multicenter study. Collaborative Osteonecrosis Group.Clin Orthop Relat Res.1999;(369):312-326. [6]Sotereanos NG,DeMeo PJ,Hughes TB,et al. Autogenous osteochondral transfer in the femoral head after osteonecrosis.Orthopedics.2008;31(2):177. [7]Liu G,Wang J,Yang S,et al.Effect of a porous tantalum rod on early and intermediate stages of necrosis of the femoral head.Biomedl Mater.2010;5(6):065003. [8]Schmitt-Sody M,Kirchhoff C,Mayer W,et al.Avascular necrosis of the femoral head: inter- and intraobserver variations of Ficat and ARCO classifications.Int Orthopaed.2008;32(3):283-287. [9]Wang LY,Fasulo D.A fast boosting-based screening method forlarge-scale association study in complex traits with genetic heterogeneity.Conf Proc IEEE Eng Med Biol Soc.2006;1:5771-5774. [10]Valafar F.Pattern recognition techniques in microarray data analysis:a survey.Ann N Y Acad Sci. 2002;980: 41-64. [11]Zardiackas LD,Parsell DE,Dillon LD,et al.Structure, metallurgy, and mechanical properties of a porous tantalum foam.J Biomed Mater Res. 2001;58(2): 180-187. [12]Bobyn JD,Stackpool GJ,Hacking SA,et al. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial.J Bone Joint Surg Br.1999;81(5):907-914. [13]Minagar S,Berndt CC,Wen C.Fabrication and Characterization of Nanoporous Niobia, and Nanotubular Tantala, Titania and Zirconia via Anodization.J Funct Biomater. 2015;6(2):153-170. [14]Whitney GA,Mera H,Weidenbecher M,et al.Methods for producing scaffold-free engineered cartilage sheets from auricular and articular chondrocyte cell sources and attachment to porous tantalum.Biores Open Access. 2012;1(4):157-165. [15]Wang Q,Zhang H,Li Q,et al.Biocompatibility and osteogenic properties of porous tantalum.Exp Ther Med.2015;9(3):780-786. [16]Ren B,Zhai Z,Guo K,et al.The application of porous tantalum cylinder to the repair of comminuted bone defects: a study of rabbit firearm injuries.Int J Clin Exp Med. 2015;8(4):5055-5064. [17]Liu ZH,Guo WS,Li ZR,et al.Porous tantalum rods for treating osteonecrosis of the femoral head.Genet Mol Res.2014;13(4):8342-8352. [18]赵德伟,谢辉,王本杰,等.带血管蒂髂骨瓣转移联合多孔钽金属棒植入治疗股骨头缺血性坏死[J].中华显微外科杂志,2014,37(1):29-34. [19]Tanzer M,Bobyn JD,Krygier JJ,et al.Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants.J Bone Joint Surg Am. 2008; 90(6):1282-1289. [20]Miao H,Ye D,Liang W,et al.Effect of Osteonecrosis Intervention Rod Versus Core Decompression Using Multiple Small Drill Holes on Early Stages of Necrosis of the Femoral Head: A Prospective Study on a Series of 60 Patients with a Minimum 1-Year-Follow-Up. Open Orthop J. 2015;9:179-184. [21]胥少汀.骨科手术并发症预防与处理[M].3版.北京:人民军医出版社,2010. [22]Malizos KN,Papasoulis E,Dailiana ZH,et al.Early results of a novel technique using multiple small tantalum pegs for the treatment of osteonecrosis of the femoral head: a case series involving 26 hips. Bone Joint Surge Br. 2012;94(2):173-178. [23]Shi J,Chen J,Wu J,et al.Evaluation of the 3D finite element method using a tantalum rod for osteonecrosis of the femoral head.Med Sci Monit. 2014;20:2556-2564. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Ma Zhijie, Li Jingyu, Cao Fang, Liu Rong, Zhao Dewei. Influencing factors and biological property of novel biomedical materials: porous silicon carbide coated with bioactive tantalum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 558-563. |
| [5] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [6] | Shi Xiaoxiu, Mao Shilong, Liu Yang, Ma Xingshuang, Luo Yanfeng. Comparison of tantalum and titanium (alloy) as orthopedic materials: physical and chemical indexes, antibacterial and osteogenic ability [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 593-599. |
| [7] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [8] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [9] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [10] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [11] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [12] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [13] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [14] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [15] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||