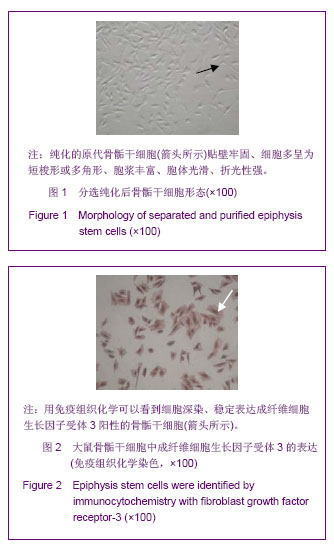
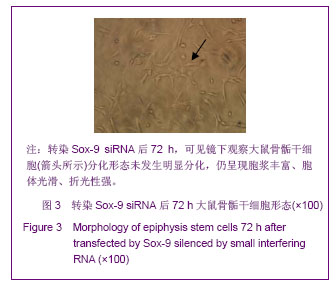
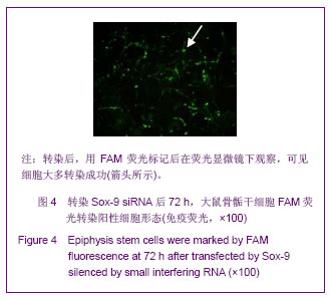
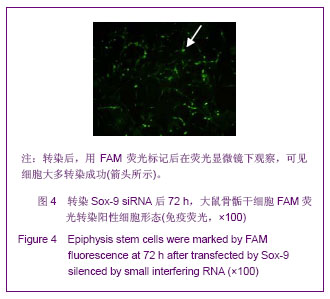
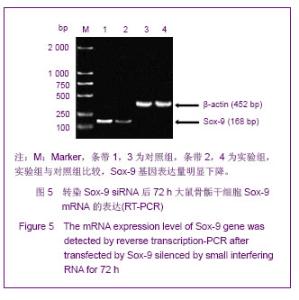
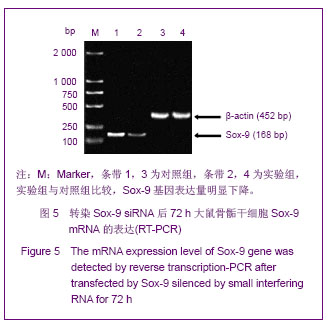
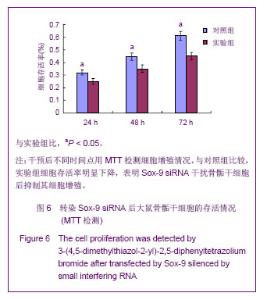
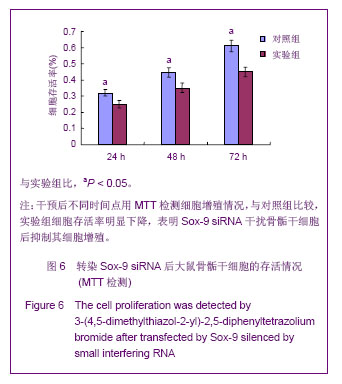
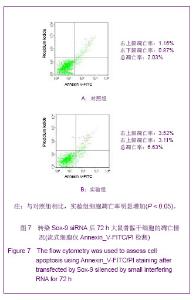
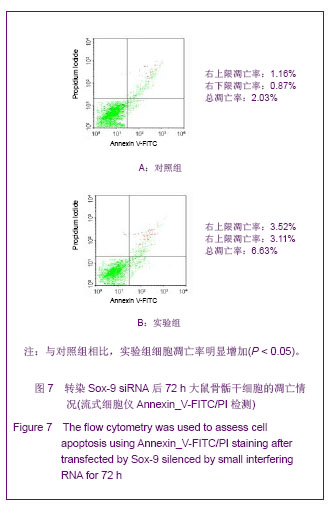
Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (40): 7068-7075.doi: 10.3969/j.issn.2095-4344.2013.40.008
Previous Articles Next Articles
Effects of Sox-9 gene silenced by small interfering RNA on proliferation and apoptosis of epiphysis stem cells
Chen Shao-jian1, Guo Feng-jin2, Wang Chun1, Gong Chen2
- 1 Department of Orthopedics, Min Dong Hospital, Fujian Medical University, Ningde 355000, Fujian Province, China; 2 Department of Orthopedics, Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan 430030, Hubei Province, China
-
Online:2013-10-01Published:2013-10-31 -
Contact:Guo Feng-jin, Professor, Doctoral supervisor, Department of Orthopedics, Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan 430030, Hubei Province, China fjguo@tjh.tjmu.edu.cn -
About author:Chen Shao-jian★, Master, Physician, Department of Orthopedics, Min Dong Hospital, Fujian Medical University, Ningde 355000, Fujian Province, China ankycsj@163.com -
Supported by:the National Natural Science Foundation of China, No. 31070831*
CLC Number:
Cite this article
Chen Shao-jian, Guo Feng-jin, Wang Chun, Gong Chen. Effects of Sox-9 gene silenced by small interfering RNA on proliferation and apoptosis of epiphysis stem cells [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(40): 7068-7075.
share this article
| [1]Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010; 16(3):305-329. [2]Peretti GM, Pozzi A, Ballis R, et al. Current surgical options for articular cartilage repair. Acta Neurochir Suppl. 2011;108: 213-219. [3]巩清波.关节软骨的损伤及其修复现状[J].中国组织工程研究与临床康复,2010,14(24):4491-4494. [4]Stoltz JF, Huselstein C, Schiavi J, et al. Human stem cells and articular cartilage tissue engineering. Curr Pharm Biotechnol. 2012;13(15):2682-2691. [5]Fini M, Pagani S, Giavaresi G, et al. Functional tissue engineering in articular cartilage repair: is there a role for electromagnetic biophysical stimulation. Tissue Eng Part B Rev. 2013;19(4):353-367. [6]Johnstone B, Alini M, Cucchiarini M, et al. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater. 2013;25:248-267. [7]Muhammad H, Schminke B, Miosge N. Current concepts in stem cell therapy for articular cartilage repair. Expert Opin Biol Ther. 2013;13(4):541-548. [8]赖建明,林建华.基因修饰骨髓间充质干细胞修复关节软骨损伤[J].中国组织工程研究,2013,(6):1107-1110. [9]罗文,范建楠,叶川.软骨组织工程学方法修复关节软骨缺损[J].中国组织工程研究,2012,16(37):7009-7014. [10]程越,田京.纳米支架与关节软骨重建[J].中国组织工程研究, 2012, 16(12):2265-2269. [11]Luo W, Fan J, Ye C. Proliferation and chondrogenic differentiation of precartilaginous stem cells in self-assembling peptide nanofiber scaffolds. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(12):1505-1511. [12]You H, Chen A, Liu T, et al. Construction of eukaryotic expression plasmid of hTGF-beta3 and its inducing effect on differentiation of precartilaginous stem cells into chondroblasts. J Huazhong Univ Sci Technolog Med Sci. 2011;31(4):524-529. [13]赵友,尹航,王昌兴,等.骨骺干细胞研究现状[J].中华中医药学刊, 2012,(2):346-348. [14]Pan YS, Ding GX, Wang J. Research on repair strategies for articular cartilage defects. Zhongguo Gu Shang. 2013;26(2): 175-178. [15]Akiyama H. Transcriptional regulation in chondrogenesis by Sox-9. Clin Calcium. 2011;21(6):845-851. [16]Tew SR, Clegg PD. Analysis of post transcriptional regulation of SOX-9 mRNA during in vitro chondrogenesis. Tissue Eng Part A. 2011;17(13-14):1801-1807. [17]Chen S, Tao J, Bae Y, et al. Notch gain of function inhibits chondrocyte differentiation via Rbpj-dependent suppression of Sox-9. J Bone Miner Res. 2013;28(3): 649-659. [18]Sugimoto Y, Takimoto A, Akiyama H, et al. Scx+/Sox-9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013; 140(11):2280-2288. [19]Cao L, Yang F, Liu G, et al. The promotion of cartilage defect repair using adenovirus mediated Sox-9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials. 2011;32(16):3910-3920. [20]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30. [21]张衣北,陈安民,郭风劲,等.骨骺干细胞的体外培养及生长特性[J].中国康复,2006;21(1):3-5. [22]游洪波,程浩.免疫磁性细胞分选技术分离纯化新生大鼠骨骺前软骨干细胞[J].中华创伤杂志,2004,20(10):33-35. [23]Hu WH, Guo FJ, Li F, et al. Construction of Sox-9 gene eukaryotic expression vector and its inductive effects on directed differentiation of bone marrow stromal cells into precartilaginous stem cells in rats. J Huazhong Univ Sci Technolog Med Sci. 2009;(3):291-295. [24]Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78(5):721-733. [25]Gobbi A, Kon E, Berruto M, et al. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years' follow-up. Am J Sports Med. 2009;37(6):1083-1092. [26]Hamanishi M, Nakasa T, Kamei N, et al. Treatment of cartilage defects by subchondral drilling combined with covering with atelocollagen membrane induces osteogenesis in a rat model. J Orthop Sci. 2013;19(4):353-367. [27]Gao SJ, Wei JC, Lu B, et al. Experimental research on repairing full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow- derived mononuclear cells in combination with micro-fracture. Zhonghua Yi Xue Za Zhi. 2012;92(35):2463-2467. [28]Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. [29]Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37(1-2):1-57. [30]Guilak F, Butler DL, Goldstein SA. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res. 2001;(391 Suppl):S295-S305. [31]El Sayed K, Marzahn U, John T, et al. PGA-associated heterotopic chondrocyte cocultures: implications of nasoseptal and auricular chondrocytes in articular cartilage repair. J Tissue Eng Regen Med. 2013;7(1):61-72. [32]Singh M, Sandhu B, Scurto A, et al. Microsphere-based scaffolds for cartilage tissue engineering: using subcritical CO(2) as a sintering agent. Acta Biomater. 2010;6(1):137-143. [33]Raub CB, Hsu SC, Chan EF, et al. Microstructural remodeling of articular cartilage following defect repair by osteochondral autograft transfer. Osteoarthritis Cartilage. 2013;21(6):860- 868. [34]Zhao Q, Wang S, Tian J, et al. Combination of bone marrow concentrate and PGA scaffolds enhance bone marrow stimulation in rabbit articular cartilage repair. J Mater Sci Mater Med. 2013;24(3):793-801. [35]Leijten JC, Georgi N, Wu L, et al. Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue Eng Part B Rev. 2013;19(1):31-40. [36]Kamei G, Kobayashi T, Ohkawa S, et al. Articular cartilage repair with magnetic mesenchymal stem cells. Am J Sports Med. 2013;41(6):1255-1264. [37]Robinson D, Hasharoni A, Cohen N, et al. Fibroblast growth factor receptor-3 as a marker for precartilaginous stem cells. Clin Orthop Relat Res. 1999;(367 Suppl):S163-175. [38]Cheng H, Chen AM , You HB. immunomagnetic indirect positive sorting of precartilaginous stem cells from neonatal rat. Huazhong Keji Daxue Xuebao (Yixue Yingdewen Ban). 2006;(6):723-724. [39]丁然,张勇,游洪波,等.人转化生长因子β3基因转染KLD-12自组装纳米肽纤维支架三维培养前软骨干细胞[J].中国组织工程研究与临床康复,2010,14(29):5339-5343. [40]You HB, Chen AM, Liu T, et al. Construction of eukaryotic expression plasmid of htgf-β3 and its inducing effect on differentiation of precartilaginous stem cells into chondroblasts. J Huazhong Univ Sci Technolog Med Sci. 2011;31(4):524-529. [41]You HB. Chondrogenesis of precartilaginous stem cells in KLD-12 self-assembling peptide nanofiber scaffold loading TGF-β3 gene. Wuhan Ligong Daxue Xuebao:Cailiao Kexueban. 2011;(4):634-640. [42]胡伟华,郭风劲,陈安民,等.新生大鼠前软骨干细胞株的体外培养分选、鉴定及永生化研究(英文)[J].中国组织工程研究与临床康复,2008,12(43):8588-8592. [43]张衣北,陈安民,郭风劲,等.Notch1信号系统对骨骺干细胞增殖与分化调控作用的初步观察[J].中华医学杂志,2005,85(48): 3430-3434. [44]周治国,李丽,郭风劲.甲状旁腺相关肽调控骨骺干细胞后的stathmin表达[J].生物骨科材料与临床研究,2010,7(1):10- 15. [45]赵守军,熊文化,郭宁峰.乏氧环境下骨骺干细胞株在软骨载体中的相容性、增殖和分化成软骨的实验研究[J].现代实用医学, 2012,24(4):375-377. [46]Kupcsik L, Stoddart MJ, Li Z, et al. Improving chondrogenesis: potential and limitations of SOX-9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A. 2010;16(6):1845-1855. [47]Hattori T, Muller C, Gebhard S, et al. SOX-9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. 2010; 137(6):901-911. [48]Nakamura Y, He X, Kato H, et al. Sox-9 is upstream of microRNA-140 in cartilage. Appl Biochem Biotechnol. 2012; 166(1):64-71. [49]Martinez-Sanchez A, Dudek KA, Murphy CL. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX-9 by microRNA-145 (miRNA-145). J Biol Chem. 2012;287(2):916-924. [50]Park J, Zhang JJ, Moro A, et al. Regulation of Sox-9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn. 2010;239(2):514-526. [51]Fanburg-Smith JC, Auerbach A, Marwaha JS, et al. Reappraisal of mesenchymal chondrosarcoma: novel morphologic observations of the hyaline cartilage and endochondral ossification and beta-catenin, Sox-9, and osteocalcin immunostaining of 22 cases. Hum Pathol. 2010; 41(5):653-662. [52]Cucchiarini M, Terwilliger EF, Kohn D, et al. Remodelling of human osteoarthritic cartilage by FGF-2, alone or combined with Sox9 via rAAV gene transfer. J Cell Mol Med. 2009; 13(8B): 2476-2488. [53]张伟凯,陈安民,郭风劲,等.大鼠骨骺干细胞免疫纯化和Sox-9基因真核表达载体的克隆构建[J].中国组织工程研究与临床康复,2008,12(12):2321-2325. [54]张树威,金伟,祝少博,等.甲状旁腺素相关蛋白亚基因对骨骺干细胞的调控[J].中国组织工程研究,2013,17(10):1814-1820. [55]Cucchiarini M, Orth P, Madry H. Direct rAAV SOX-9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J Mol Med (Berl). 2013;91(5):625-636. |
| [1] | Tan Xinfang, Guo Yanxing, Qin Xiaofei, Zhang Binqing, Zhao Dongliang, Pan Kunkun, Li Yuzhuo, Chen Haoyu. Effect of uniaxial fatigue exercise on patellofemoral cartilage injury in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-6. |
| [2] | Wu Cong, Jia Quanzhong, Liu Lun. Relationship between transforming growth factor beta1 expression and chondrocyte migration in adult articular cartilage after fragmentation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1167-1172. |
| [3] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [4] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [5] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [6] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [7] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [8] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [9] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [10] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [11] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [12] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [13] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [14] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [15] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||