Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (36): 6481-6488.doi: 10.3969/j.issn.2095-4344.2013.36.016
Previous Articles Next Articles
Molecular imaging for tracking transplanted embryonic stem cells in the treatment of acute liver injury
Yao Xin-peng1,2, Xu Yang2, Zhang Lu1, Leng Liang2, Su Wei-jun2, Wang Li-na2, Tong Ling-ling2, Li Zong-jin2, Kong De-ling1
- 1Key Laboratory of Bioactive Materials, Ministry of Education, Nankai University, Tianjin 300071, China; 2School of Medicine, Nankai University, Tianjin 300071, China
-
Received:2013-02-16Revised:2013-02-25Online:2013-09-03Published:2013-09-03 -
Contact:Li Zong-jin, M.D., Professor, School of Medicine, Nankai University, Tianjin 300071, China zongjinli@nankai.edu.cn Xu Yang, M.D., Lecturer, School of Medicine, Nankai University, Tianjin 300071, China yangxu@nankai.edu.cn -
About author:Yao Xin-peng☆, Studying for doctorate, Key Laboratory of Bioactive Materials, Ministry of Education, Nankai University, Tianjin 300071, China; School of Medicine, Nankai University, Tianjin 300071, China yxpeng8744@163.com -
Supported by:the National Basic Research Key Program of China, No. 2010CB94500*
CLC Number:
Cite this article
Yao Xin-peng, Xu Yang, Zhang Lu, Leng Liang, Su Wei-jun, Wang Li-na, Tong Ling-ling, Li Zong-jin, Kong De-ling. Molecular imaging for tracking transplanted embryonic stem cells in the treatment of acute liver injury[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(36): 6481-6488.
share this article
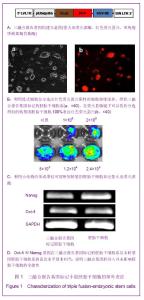
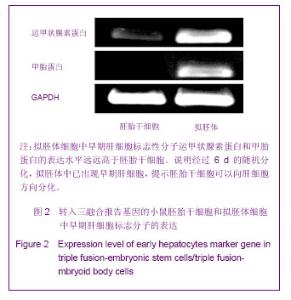
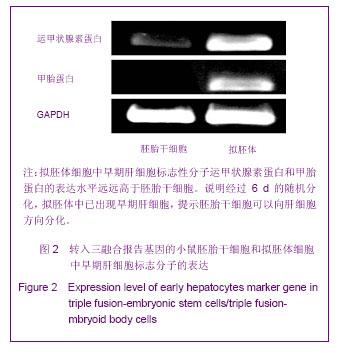
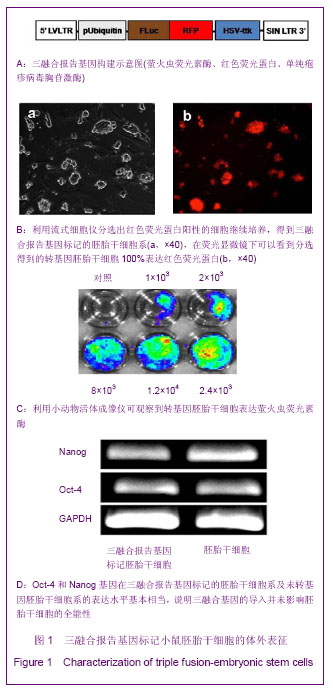
2.1 三融合报告基因标记的小鼠胚胎干细胞系的建立与全能性鉴定结果 如图1A所示,构建了pUbiquitin启动子启动的,萤火虫荧光素酶、红色荧光蛋白以及单纯疱疹病毒胸苷激酶三融合报告基因,利用慢病毒系统将其导入D3胚胎干细胞中,利用流式细胞仪分选出红色荧光蛋白阳性的细胞继续培养,得到三融合报告基因标记的胚胎干细胞系,在荧光显微镜下可以看到分选得到的转基因胚胎干细胞100%表达红色荧光蛋白,见图1B。向细胞培养基中加入萤火虫荧光素酶的底物D-luciferin,利用小动物活体成像仪可观察到转基因胚胎干细胞发出的荧光信号,如图1C。为了考察慢病毒介导基因转导过程是否会对胚胎干细胞的全能性造成影响,实验利用RT-PCR的方法鉴定未转基因的D3胚胎干细胞和D3转基因胚胎干细胞中胚胎干细胞的标志分子Oct-4和Nanog基因的表达情况[13-14],结果显示上述2种基因在2株细胞系的表达水平基本相当,一定程度上说明三融合基因的导入并未影响胚胎干细胞的全能性,见图1D。"

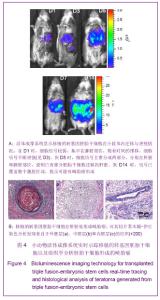
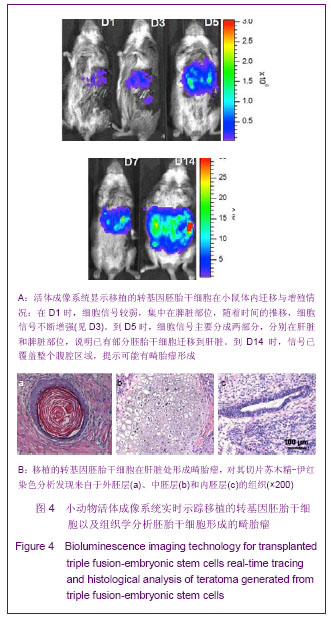
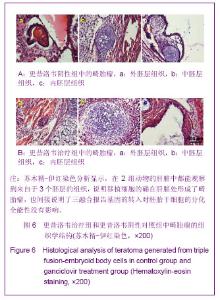
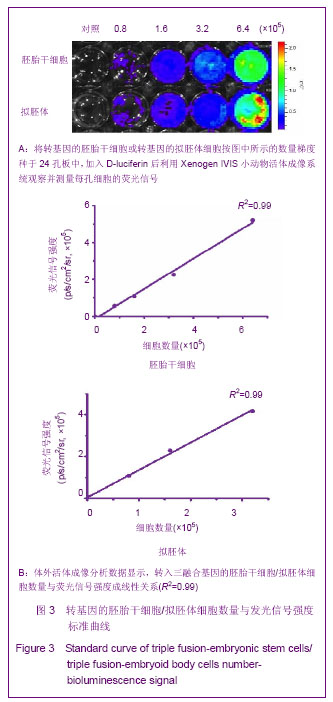
2.3 细胞数量与荧光信号强度标准曲线绘制结果 为了建立细胞数量与荧光信号强度的标准曲线,将转基因的胚胎干细胞或转基因的拟胚体细胞按图3A所示的数量梯度种于24孔板中,加入D-luciferin后利用Xenogen IVIS小动物活体成像系统观察并测量每孔细胞的荧光信号如图3A,绘制细胞数量-荧光信号标准曲线,反应变量关联度的R2值均大于0.99,说明细胞数量与荧光信号强度成正相关,如图3B。 2.4 转基因胚胎干细胞体内的迁移及增殖情况 转基因胚胎干细胞注射后,从第1天(D1)起,每2 d对移植细胞进行1次成像,直至D14。如图4A所示,在D1时,细胞信号较弱,集中在脾脏部位,随着时间的推移,细胞信号不断增强(见D3),说明胚胎干细胞在体内不断增殖。到D5时,除了观察到细胞信号进一步增强外,同时观察到细胞信号主要分成两部分,分别在肝脏和脾脏部位,说明已有部分胚胎干细胞迁移到肝脏。随着时间的推移,发现肝脏处的信号逐渐增强,到D14时,信号已覆盖整个腹腔区域,提示可能有畸胎瘤形成。 D14时,将小鼠肝脏取材,进行石蜡切片分析。苏木精-伊红染色结果显示,在小鼠肝组织中发现来自于3个胚层的组织如外胚层的神经管组织、中胚层的软骨组织、内胚层的上皮组织,见图4B,说明胚胎干细胞在肝脏处形成了畸胎瘤。上述现象说明移植的转基因胚胎干细胞可以从脾脏迁移到肝脏,并在肝脏处恶性增殖形成了畸胎瘤。"

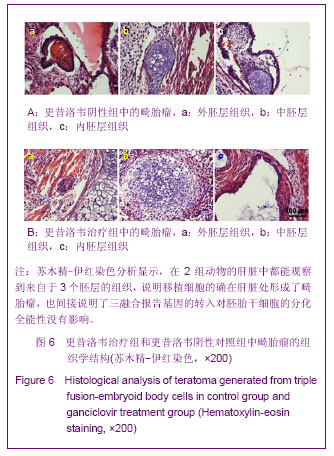
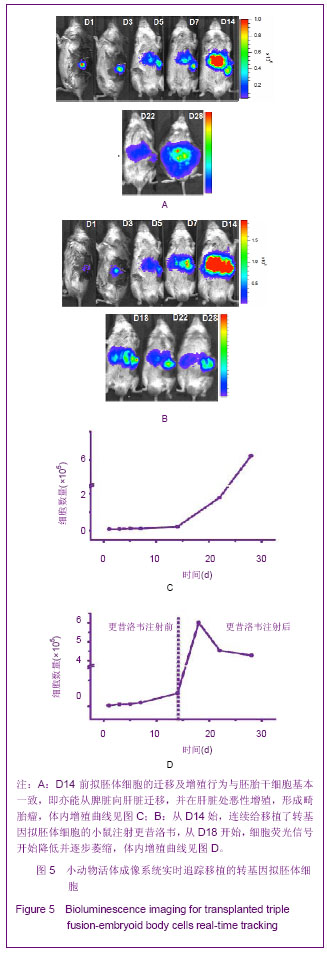
2.5 转基因拟胚体细胞移植后在体内的行为 见图5。 鉴于直接移植未分化的转基因胚胎干细胞组畸胎瘤的形成,移植来自于分化了6 d的转入三融合报告基因的拟胚体细胞,以期避免畸胎瘤的形成。从图5A中观察到,D14前拟胚体细胞的迁移及增殖行为与胚胎干细胞基本一致,即亦能从脾脏向肝脏迁移,并在肝脏处恶性增殖,形成畸胎瘤。图5C显示了拟胚体细胞移植后在体内的数量变化曲线。 单纯疱疹病毒胸苷激酶是种自杀基因,可以与其底物更昔洛韦作用,诱导细胞死亡[12]。对于移植转基因拟胚体细胞发生恶性增殖的小鼠,期望通过连续腹腔注射更昔洛韦将畸胎瘤清除。从D14始,连续给移植了转基因拟胚体细胞的小鼠注射更昔洛韦,从D18开始,细胞荧光信号开始降低并逐步萎缩,如图5B。至D28时,与未注射更昔洛韦组相比(图5A),注射组细胞荧光信号已降至较低水平(图5B),说明畸胎瘤细胞已被部分清除。 "
| [1] Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981; 292 (5819):154-156. [2] Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008; 451(7181):937-942. [3] Schroeder T. Imaging stem-cell-driven regeneration in mammals. Nature. 2008; 453(7193):345-351. [4] Close DM, Hahn RE, Patterson SS, et al. Comparison of human optimized bacterial luciferase, firefly luciferase, and green fluorescent protein for continuous imaging of cell culture and animal models. J Biomed Opt. 2011; 16(4):047003. [5] Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011; 8(11):677-688. [6] Zanzonico P. Principles of nuclear medicine imaging: planar, SPECT, PET, multi-modality, and autoradiography systems. Radiat Res. 2012; 177(4):349-364. [7] Kremer AE, Elferink RPJO, Beuers U. Pathophysiology and current management of pruritus in liver disease. Clin Res Hepatol Gastroenterol. 2011; 35(2):89-97. [8] Snykers S, De Kock J, Rogiers V, et al. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem cells. 2009; 27(3):577-605. [9] Yamada T, Yoshikawa M, Kanda S, et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002; 20(2): 146-154. [10] Kanda S, Shiroi A, Ouji Y, et al. In vitro differentiation of hepatocyte-like cells from embryonic stem cells promoted by gene transfer of hepatocyte nuclear factor 3 beta. Hepatol Res. 2003; 26(3):225-231. [11] Chinzei R, Tanaka Y, Shimizu-Saito K, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002; 36(1):22-29. [12] Cao F, Drukker M, Lin S, et al. Molecular Imaging of Embryonic Stem Cell Misbehavior and Suicide Gene Ablation. Cloning Stem Cells. 2007; 9(1):107-117. [13] Zaehres H, Lensch MW, Daheron L, et al. High-efficiency RNA interference in human embryonic stem cells. Stem cells. 2005; 23(3):299-305. [14] Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003; 113(5):643-655. [15] Moriya K, Yoshikawa M, Saito K, et al. Embryonic stem cells develop into hepatocytes after intrasplenic transplantation in CCl4-treated mice. World J Gastroenterol. 2007;13(6):866-873. [16] Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003; 108(11):1302-1305. [17] Chen IY, Wu JC. Cardiovascular Molecular Imaging Focus on Clinical Translation. Circulation. 2011; 123(4):425-443. [18] Oostendorp M, Douma K, Wagenaar A, et al. Molecular magnetic resonance imaging of myocardial angiogenesis after acute myocardial infarction. Circulation. 2010; 121(6): 775-783. [19] Wang J, Najjar A, Zhang S, et al. Molecular Imaging of Mesenchymal Stem Cell Mechanistic Insight Into Cardiac Repair After Experimental Myocardial Infarction. Cir Cardiovasc Imaging. 2012; 5(1):94-101. [20] Li Z, Wu JC, Sheikh AY, et al. Differentiation, survival, and function of embryonic stem cell-derived endothelial cells for ischemic heart disease. Circulation. 2007; 116(11):I46-I54. [21] Ebert SN, Taylor DG, Nguyen HL, et al. Noninvasive tracking of cardiac embryonic stem cells in vivo using magnetic resonance imaging techniques. Stem Cells. 2007; 25(11): 2936-2944. [22] Hwang DW, Kang JH, Jeong JM, et al. Noninvasive in vivo monitoring of neuronal differentiation using reporter driven by a neuronal promoter. Eur J Nucl Med Mol Imaging. 2008; 35(1):135-145. [23] Bossolasco P, Cova L, Levandis G, et al. Noninvasive near-infrared live imaging of human adult mesenchymal stem cells transplanted in a rodent model of Parkinson's disease. Int J Nanomedicine. 2012; 7:435-447. [24] De Vocht N, Reekmans K, Bergwerf I, et al. Multimodal imaging of stem cell implantation in the central nervous system of mice. J Vis Exp. 2012; (64):e3906. [25] Ozdemir M, Attar A, Kuzu I, et al. Stem cell therapy in spinal cord injury: in vivo and postmortem tracking of bone marrow mononuclear or mesenchymal stem cells. Stem Cell Rev. 2012; 8(3):953-962. [26] Wang J, Tian M, Zhang H. PET molecular imaging in stem cell therapy for neurological diseases. Eur J Nucl Med Mol Imaging. 2011; 38(10):1926-1938. [27] Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007; 317 (5845):1767-1770. [28] Barrett O, Sottocornola R, Lo Celso C. In vivo imaging of hematopoietic stem cells in the bone marrow niche. Methods Mol Biol. 2012; 916:231-242. [29] McCracken MN, Gschweng EH, Nair-Gill E, et al. Long-term in vivo monitoring of mouse and human hematopoietic stem cell engraftment with a human positron emission tomography reporter gene. Pro Natl Acad Sci USA. 2013; 110(5): 1857-1862. [30] Rosland GV, Svendsen A, Torsvik A, et al. Long-term Cultures of Bone Marrow-Derived Human Mesenchymal Stem Cells Frequently Undergo Spontaneous Malignant Transformation. Cancer Res. 2009; 69(13):5331-5339. [31] Wang Y, Huso DL, Harrington J, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005; 7(6): 509-519. [32] Monnier D, Gourmelon P, Gorin NC. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010; 115(8): 1549-1553. [33] Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci. 2012; 1266:107-117. [34] Goldring CE, Duffy PA, Benvenisty N, et al. Assessing the safety of stem cell therapeutics. Cell Stem Cell. 2011; 8(6):618-628. [35] Gropp M, Shilo V, Vainer G, et al. Standardization of the Teratoma Assay for Analysis of Pluripotency of Human ES Cells and Biosafety of Their Differentiated Progeny. PLoS One. 2012;7(9):e45532. doi: 10.1371/journal.pone.0045532. [36] Teramoto K, Hara Y, Kumashiro Y, et al. Teratoma formation and hepatocyte differentiation in mouse liver transplanted with mouse embryonic stem cell-derived embryoid bodies. Transplant Proc. 2005; 37(1):285-286. [37] Bukong TN, Lo T, Szabo G, et al. Novel developmental biology-based protocol of embryonic stem cell differentiation to morphologically sound and functional yet immature hepatocytes. Liver Int. 2012; 32(5):732-741. [38] Li HY, Chen Y, Chen YJ, et al. Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials. 2011; 32(26):5994-6005. [39] Woo DH, Kim SK, Lim HJ, et al. Direct and Indirect Contribution of Human Embryonic Stem Cell-Derived Hepatocyte-Like Cells to Liver Repair in Mice. Gastroenterology. 2012; 142(3):602-611. [40] Cai J, Zhao Y, Liu Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007; 45(5):1229-1239. |
| [1] | Wang Jian-ji, Yang Long, Li Jing, Sun Qi, Zuo Wei-min, Ren Qi-feng, Sun Yu, Wu Zhan-yu, Zou Qiang, Ma Min-xian, Ye Chuan. Development and application of special-purpose grafter by femoral head decompression combined with bone marrow mesenchymal stem cells transplantation based on three-dimensional printing technology [J]. Chinese Journal of Tissue Engineering Research, 2016, 20(44): 6636-6642. |
| [2] | Zhou Chang-yan, Zhou Qing-huan, Bian Jing, Chen Ke, Chen Wen. Bone marrow mesenchymal stem cells combined with calcium phosphate cement to repair articular cartilage defects in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(8): 1195-1199. |
| [3] | Xu Xiang, Yin He-ping. Platelet-rich plasma accelerates the proliferation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2144-2148. |
| [4] | Ma Fa-ku, Wang Huan, Liu Bin, Yang Yan-li, Su Qin-jun, Qian Zhen, Dong Liang. Expression of CD44+/C-myc+ cancer stem cells and its relationship with the prognosis of patients in colorectal tumors [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2161-2166. |
| [5] |
Chen Ping, Wang Jian.
Enrichment of lung cancer stem cells and expression of related markers
[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2167-2171.
|
| [6] | Fan Zhi-gang, Fan Hong-liang. Oxidative stress response in diabetic nephropathy rats following injection of embryonic stem cells via the tail vein [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2199-2204. |
| [7] | Wang Yong, Zhao Wei, Feng Jian-zhou, Chen Xiao-chun. Nerve growth factor-modified adipose derived stem cells for repair of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2224-2229. |
| [8] | Sha Wen-qiong, She Rui-lian, Wang Zi-neng, Ke Ru. Ultrastructure and phagocytotic function of human placental mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2230-2235. |
| [9] | Du Qing-hua, Cao Jun-kai, Dong Xi-xi, E Ling-ling, Wei Li-jun. Osteogenic differentiation of pluripotent stem cells induced by akermanite extracts [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2236-2242. |
| [10] | Wu Yan, Huang Lan . Bone morphogenetic protein 9-induced osteogenic differentiation of dental follicle cells in vitro [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2255-2260. |
| [11] | Rao Li-jia, Li Qi-meng, Li Jin-ling, Xu Qiong. Expression pattern of ten-eleven translocation family during differentiation of human dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2261-2266. |
| [12] | Gao Zhuo-yue, Liu Yong-qi, He Jian-xin, Wu Zhi-wei, Luo Ya-li, Su Yun, Zhang Li-ying, Zhang Qi, Wu You-ming, Zhou Ni-na. Regulatory effects of warming yang and invigorating qi treatment on the inflammatory balance and genetic stability of bone marrow mesenchymal stem cells under tumor microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2267-2272. |
| [13] | Wang Fang, Chen Shao-wei. In vitro culture of embryos and establishment of embryonic stem cell lines [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2273-2277. |
| [14] |
Du Wei-bin, Quan Ren-fu, Zheng Xuan, Wang Tuo.
Hair follicle stem cells promote the healing of skin wound
|
| [15] | Du Hua, Shi Ying-xu . Bone marrow microenvironment controls the “fate” of hematopoietic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(14): 2283-2290. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||