Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (28): 4465-4473.doi: 10.3969/j.issn.2095-4344.2305
Previous Articles Next Articles
A drug-loading system for electrospinning wound repair: component selection and construction strategy
Liu Yanhua, Zhu Zhou, Wan Qianbing
- Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2019-11-05Revised:2019-11-13Accepted:2019-12-19Online:2020-10-08Published:2020-08-31 -
Contact:Wan Qianbing, Professor, Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Liu Yanhua, Master candidate, Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
Supported by:the Key Research and Development Project of Science Technology Department of Sichuan Province, No. 2018SZ0037
CLC Number:
Cite this article
Liu Yanhua, Zhu Zhou, Wan Qianbing. A drug-loading system for electrospinning wound repair: component selection and construction strategy[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4465-4473.
share this article
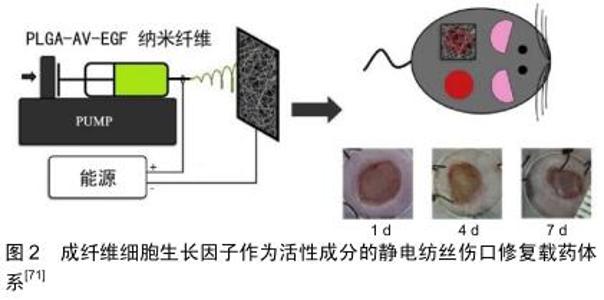
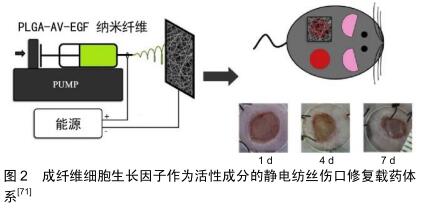
2.2 静电纺丝的基础:聚合纤维 在静电纺丝纤维膜中,电纺纤维原料决定了纺丝膜体系的基本性质和作用。一般来说,形成基底膜的纤维原料是聚合纤维,主要包括生物来源聚合纤维和化学合成聚合纤维2类。文章将依照这2个分类,选取在伤口软组织愈合过程中发挥作用的一些常用聚合纤维原料进行阐述。 2.2.1 生物型聚合纤维 现代生产技术提取的生物来源原料纤维拥有良好的生物相容性,大多数能提供类似于生物微环境的三维立体结构,并在一定程度上参与信号传导。然而,单一使用部分生物纤维应用有可能会引发一些不必要的过敏反应[15]。另外,在体液环境下生物纤维的机械强度和稳定性都不及合成纤维,这也在一定程度上限制了生物纤维的运用。 胶原蛋白:是细胞外基质的重要成分之一,广泛存在于皮肤,骨骼,肌腱等结缔组织中。胶原蛋白直径介于50-500 nm之间,呈纤维状结构排列,是细胞外基质中三维网络的骨架成分之一。在实际的纺丝运用中,胶原纤维通常需要后续修饰以增加其在水溶液中的结构稳定性[16-18]。同时生产中还需要特别注意仪器高压作用引起的胶原蛋白构象变性[19]。为了获得具有刚性结构的纳米纤维基质,通常将胶原与其他合成聚合物如聚己内酯共混作为原料电极液[20]。胶原的良好的特异性网状三维结构可作为细胞攀附爬行的支架,有助于细胞附着,生长和增殖,从而保证伤口区域的组织恢复[19]。 壳聚糖:是一种生物来源的可降解多糖。由于壳聚糖及其衍生物对真菌和细菌的固有抗菌特性,而被用于伤口敷料材料[16,21]。在实际纺丝过程中因为壳聚糖溶液的黏度很高,作为单一聚合物溶液不容易静电纺丝,通常会加入聚合物如聚氧化乙烯或聚乙烯醇以降低溶液黏度混纺来达到理想的纺丝效果[22-23]。研究表明,单独的聚乳酸纳米纤维基质不能抑制细菌生长(金黄色葡萄球菌和大肠杆菌),但随着壳聚糖或季铵化壳聚糖的加入,可检测到这两种细菌的生长受到明显抑制[24]。这种抗菌活性可能是由于带正电荷的壳聚糖和带负电荷的细菌胞膜之间的离子相互作用产生的。此外,壳聚糖的化学结构与细胞外基质中具有止血活性的糖胺聚糖具有很高的相似性[25],因此壳聚糖的止血作用也可能是促伤口愈合的一个机制。潜在的抗菌性能及止血性能使得壳聚糖成为了静电纺丝伤口辅料制作中的重要选择之一。 透明质酸:是一种天然的线性多糖,是结缔组织胞外基质的主要成分,具有独特的黏弹性、高保水性和优良的生物相容性和生物可降解性,使透明质酸及其衍生物得到了广泛的应用,例如制作化妆品、药物输送系统、伤口敷料、用于关节炎治疗和组织工程支架等[26-28]。BAHMAN-HOSSEINZADEH等[29]将透明质酸纤维静电纺丝与普通商用凝胶和常规伤口敷料进行对比,实验结果显示该透明质酸材料极大地加速了愈合速度,并且减少了伤口区域的炎症反应,表明其对伤口愈合更加有利。 其他:其他纤维,比如具有优异止血功能的藻酸盐多糖纤维[30]、纤维素[31]、聚甲基丙烯酸磺基甜菜碱[32],各种动植物提取蛋白如大豆蛋白[33]、玉米蛋白[34-35]、角蛋白[36]、丝蛋白等也都在制作促进伤口软组织愈合的静电纺丝纤维膜时有所运用[37]。 2.2.2 合成型聚合纤维 合成型纤维能够提供优异的机械强度和稳定性,并且在纺丝过程中也表现出对各种参数条件更强的适应性,因此常作为基础材料参与构建缓释控释体系。但合成纤维生理作用单一,还可能存在合成原料/溶剂的残留,其生物相容性不如生物型纤维。下文列举将列举几种静电纺丝中常用的合成纤维。 聚己内酯:是半结晶且可生物降解的聚酯。在生理条件下,聚己内酯可通过聚合物中的酯键水解并降解成无毒产物。鼠皮下植入实验显示,使用聚己内酯制备的胶囊状物体内缓慢降解的过程中均未检测到毒性反应[38]。这种良好的生物性能使聚己内酯材料非常适合用于制作长期植入式医疗用品。ZHU等[39]制备的聚己内酯静电纺丝载药系统能够有效改善血管再生,在促进伤口再生愈合过程中发挥了良好作用。聚己内酯优越的物理机械特性仍使其在静电纺丝生产中占有重要位置[40-42]。 聚乙烯醇:是一种水溶性聚合物,这种特性决定了聚乙烯醇纤维在水溶液中不能稳定存在[43]。对于具有较多渗出物的伤口,聚乙烯醇这种纤维需要进一步修饰以保持其结构稳定性。有研究通过甲醇、乙醇、高温3种方法分别处理聚乙烯醇纤维来增强其稳定性,并且验证了这些处理方法的效果[44]。其中效果最好的是高温法,处理后纳米纤维聚乙烯醇基体可在水溶液条件下保留其标准结构达96 h。因此,受益于自身良好的生物相容性和降解性,聚乙烯醇常被用于稳定药物释放或与其他纤维联用以改善材料整体的降解性能[45-47]。 聚乳酸-乙醇酸:是聚乳酸聚乙醇酸聚合产出的共聚物。一般情况下,聚乳酸-乙醇酸生物相容性良好,可通过酯键的水解代谢为水和二氧化碳。聚乳酸-乙醇酸具有降解时间的可控性等特点。研究表明,合成过程中较高的丙交酯(聚乳酸原料之一)含量会延长共聚物的降解期,而化合物末端的酸性基团则会导致聚乳酸-乙醇酸更快地降解[48]。因此,通过聚乳酸、聚乙醇酸比例和聚合程度的调整,聚乳酸-乙醇酸的降解期可以短至数周,也可以长达数年,这种特别的可控性也让聚乳 酸-乙醇酸成为静电纺丝中常用的基础纤维之一[49-50]。另一方面,也有报道指出聚乳酸-乙醇酸纤维在生物环境条件实验中发生纤维收缩的问题,需要引起注意[51]。 其他:合成聚合物在静电纺丝应用领域使用很广泛,除了上文已经提及的3种常用的纤维之外,聚乙烯吡咯烷酮[52]、聚氨酯[53]、3-羟基丁酸酯-共-3-羟基戊酸酯[54]、聚醚砜和聚环氧乙烷等聚合纤维也被用以制造促伤口愈合的纳米纤维原料[55-56]。 2.2.3 生物型与合成型聚合物复合纤维 值得注意的是,因为伤口愈合的复杂需求,静电纺丝伤口修复载药体系的纤维有时不是孤立的组分[14]。基于生物型纤维易于降解和生物相容性优异的优点[57],搭配合成型纤维的可调控性和可靠的机械强度[39,48],综合两类纤维是许多研究者的选择。例如,将聚己内酯聚合纤维良好的机械性能和透明质酸优异的吸水性结合起来,CHANDA等[26]构建了一个复合纤维组成的伤口辅料,在促进细胞黏附和迁移方面都观察到了优异的效果;与之相似的将聚己内酯与胶原[20]、壳聚糖等生物型聚合物和用的静电纺丝材料也均有报道[58]。另一方面,加入易调控降解时间的合成型聚合物(如聚乳酸-乙醇酸)用以改良生物性纤维的综合性能,在静电纺丝伤口辅料领域也较为常见:诸如聚乳酸-乙醇酸与芦荟纤维合用纺丝获得具有良好的伤口促进能力的伤口屏障[59],或是运用纤维素和聚乳 酸-乙醇酸混合的基质负载活性药物以加速糖尿病足溃疡愈合等[60]。因此,静电纺丝载药体系的纤维基础可以是单一的生物型或合成型聚合物组成,也可以根据材料技术、负载药物和临床需求的作出综合的选择。 2.3 静电纺丝载药体系的生物活性成分 在促进软组织生长的静电纺丝载药体系中,除了基底作用的聚合纤维,更加重要的是作为核心的负载药物或生物成分。静电纺丝纤维提供了优异的载药条件,使得多种活性成分能够嵌入纤维结构中或者固位于纤维表面形成载药体系。小分子抗生素分子、大分子的肽类物质,甚至一些满足特定条件的细胞都可作为“药物”加入并构成载药体系。后文将从微观尺寸出发,按照种类介绍现在常用于促伤口愈合静电纺丝载药体系的活性成分。 2.3.1 细胞活性成分 细胞活性成分指参与纤维负载体系的是细胞或类细胞整体,即整个细胞的复杂结构作为一个综合作用的有效成分。由于细胞运用的复杂性,现学者们主要在干细胞运用上进行尝试性研究。 干细胞类:由于存在病毒感染和诱导自体免疫排斥反应的风险,负载干细胞的静电纺丝纤维膜现仅用于大面积烧伤患者的恢复中[61]。有报道在静电纺丝纤维膜上培养并引导分化干细胞,再将得到相关的皮肤细胞(角质形成细胞或成纤维细胞)用于伤口愈合。干细胞治疗核心是通过干细胞和诱导的细胞提供复杂的信号分子,从而促进自然伤口愈合的过程。有研究者已经用聚左旋乳酸/胶原、聚左旋乳酸-己内酯共聚物/多聚体或聚乙烯醇/明胶等构成的纤维支架培养干细胞,以期在伤口愈合中发挥作用[62-64]。这项技术的实际运用条件非常苛刻,不仅要求特定的细胞诱导剂的作用,还要在人体内诸多生理环境难以控制的情况下达到预期的细胞作用效果[65-68]。因此现在这类材料在临床上只有极少的运用。 其他细胞活性成分:与负载干细胞的电纺载药系统的现状类似,负载富血浆血小板的纤维也是主要处于研究阶段。活化的血小板一方面可以提供凝血前期细胞信号,介导整个凝血过程的进行;另一方面可以分泌、活化或者诱导生成与血管内皮细胞,成纤维细胞,皮肤角质细胞生长繁殖有关的细胞因子,比如血管内皮生长因子、成纤维细胞生长因子2、血小板衍生生长因子、肝细胞生长因子、碱性成纤维细胞生长因子、结缔组织生长因子等[69-70]。除此之外,用质粒携带编码生长因子片段的生物活性成分也被用于促伤口愈合的电纺纤维膜中[71],见图2。 "
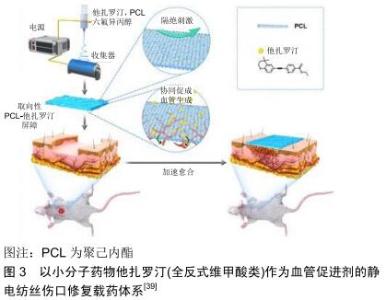
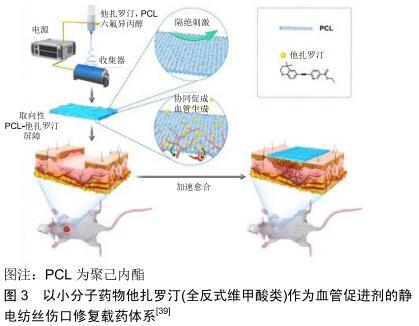
2.3.2 大分子活性成分 大分子活性成分主要是指生物细胞来源的大分子蛋白类有效成分。这种活性分子功能较为多样,在适宜条件下也能较好地模拟生物体内伤口愈合的生理过程,是非常优异的活性成分。但由于大分子蛋白类物质的不稳定性,这类活性成分的运用也受到诸多外界技术条件的限制,目前主要使用的大分子活性成分包括生长因子和生物酶类。 生长因子类:生长因子在调节伤口软组织愈合过程中起关键作用。创伤发生后,大量生长因子从角质形成细胞、成纤维细胞、血小板和巨噬细胞中释放,介导细胞产生应对行为。基于生长因子对于伤口愈合的重要作用,它们常被作为活性物质加入到静电纺丝体系中。比如ITXASO研究团队用负载表皮生长因子的静电纺丝载药系统培养成纤维细胞,显著提升了细胞的增殖能力,加速了伤口闭合和再上皮化进程[72]。成纤维细胞生长因子也可被嵌入纳米纤维中,得到一个缓释的核壳载药体系;这个载药体系可有效降低药物的突释,达到成纤维细胞生长因子控释的效果。应用该材料对糖尿病大鼠模型进行体内实验显示[73],碱性成纤维细胞生长因子负载的纤维膜组大鼠伤口比对照组的愈合更快。其他的细胞生长因子,如血管内皮生长因子也在静电纺丝载药体系中有所提及,用于促进伤口血运的重建,从而达到促进伤口愈合的效果[74]。生长因子直接而有效的促进愈合作用[72],使其成为静电纺丝伤口修复载药体系的大分子活性成分中最为常见的活性分子。 酶类:溶菌酶又称胞壁质酶或N-乙酰胞壁质聚糖水解酶,是一种能水解致病菌中黏多糖的碱性酶,主要通过破坏细胞壁中的N-乙酰胞壁酸和N-乙酰氨基葡糖之间的β-1,4糖苷键,使细胞壁不溶性黏多糖分解成可溶性糖肽,导致细胞壁破裂内容物逸出而使细菌溶解。溶菌酶还可与带负电荷的病毒蛋白直接结合,与DNA、RNA、脱辅基蛋白形成复盐使病毒失活。因此,该酶具有抗菌、消炎、抗病毒等作用[75]。CHARERNSRIWILAIWAT等[76]制备了封装溶菌酶的壳聚糖/聚乙烯醇静电纺丝载药系统,其中壳聚糖和溶菌酶作为共同抗菌有效成分发挥作用,实验表明对比空白对照组,该静电纺丝材料可以促进成纤维细胞的增殖并加速伤口愈合。 2.3.3 小分子活性成分 不同于大分子活性成分,尺寸更小的小分子成分大多是有特定作用的无机分子。虽然在生物模拟作用上逊于大分子蛋白,但是这一类别的成分化学成分相比生物大分子更稳定,作用指向更加明确,也是静电纺丝负载体系中常见的活性成分。 抗生素类:考虑到抗生素的抗菌谱、溶解性及静电纺丝过程中变性等情况,用于伤口愈合纤维膜活性成分中最常见的抗生素是环丙沙星类药物。利用静电纺丝技术、聚二乙二醇二甲基醚甲基丙烯酸酯和聚(乳酸-ε-ε-己内酯)共聚构成的热敏电纺载药系统,可通过热反应释放有效成分环丙沙星,显著降低伤口的感染概率,促进伤口愈合[77]。LIU等[49]则以聚乳酸-乙醇酸为纺丝基础,通过透明质酸微粒的亲水性改性,用以负载环丙沙星构成新型的伤口敷料,结果表明其优异的抗菌能力为伤口愈合提供了保障,到达了维持伤口区域稳定,促进愈合的效果。 另一种常用的小分子活性抗生素是盐酸米诺环素。ALHUSEIN等[78]评估了负载盐酸米诺环素的聚乙烯-乙酸乙烯酯/聚己内酯纤维膜的抗菌能力,该载药体系中抗生素持续释放,可有效防止金黄色葡萄球菌细菌膜形成并杀死菌体,达到促进伤口闭合的作用。 二甲基草酰甘氨酸:是一种小分子的非特异性抑制脯氨酰羟化酶,具有细胞渗透性。二甲基草酰甘氨酸通过抑制低氧诱导因子1α的分解,创造一种类似于缺氧的细胞微环境,在这种微缺氧的环境下,血管再生和纤维再生被活化,从而使修复速度加快[79]。ZHANG等[80]用聚己内酯和二甲基草酰甘氨酸共混静电纺丝得到载药体系纤维膜,并对该纤维膜的促伤口愈合效应进行了研究。实验以大鼠伤口为研究模型,得到结果为电纺聚己内酯/二甲基草酰甘氨酸纤维膜一方面加速了伤口愈合,另一方面能调控生长因子(胰岛素样生长因子1、表皮生长因子和神经生长因子)、抗炎因子(转化生长因子β1和白细胞介素)增加表达,降低伤口周围的炎症反应,多方面加强组织愈合。另外,同样以二甲基草酰甘氨酸作为活性成分,一种多孔的聚乳酸-乙醇酸电纺载药体系也被证实对伤口愈合具有积极作用[81]。 全反式维甲酸:又称视黄酸、维生素甲酸、维甲酸等,是动物体内维生素A的代谢中间产物,其一般药理作用在于激活视黄酸受体来调节促进上皮细胞分化与生长,维持上皮组织的正常角化过程。全反式维甲酸对糖尿病小鼠难愈伤口的治疗作用已得到了研究肯定[82]。LI等[83]将维生素A和维生素E混合于明胶纤维溶液中进行共混静电纺丝,这使得维生素A和维生素E的不稳定衍生物有效成分(维甲酸和维生素E琥珀酸酯)能发挥长效稳定的作用。 作为不良反应更小、更稳定的第3代全反式维甲酸活性成分,他扎罗汀也是值得关注的小分子活性成分之一。基于这种小分子活性成分构建的构建聚己内酯/他扎罗汀的静电纺丝载药体系,能够有效促进伤口区域的血管增殖和联合,使相关组织再生速度加快,最终促进伤口愈合[39],见图3。 "

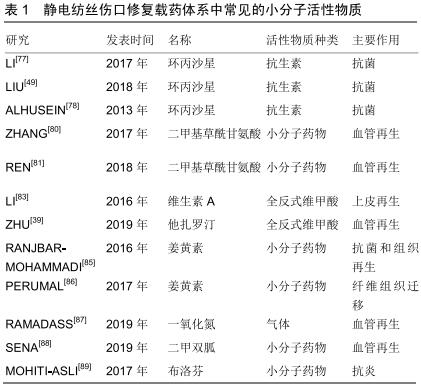
姜黄素:姜黄素具有广泛的生物学特性,例如抗炎、抗氧化和抗菌活性。因此姜黄素也作为一种活性成分用于加速伤口愈合。现有研究报告称姜黄素能有效对抗氧化应激反应,加速伤口的愈合[84]。RANJBAR-MOHAMMADI等[85]研究以聚己内酯为基础构建了负载姜黄素的电纺伤口敷料,有效发挥了抗菌作用,并促进了纤维细胞增殖、胶原蛋白沉积及完整的早期上皮层再生。除了直接的增殖促进作用外,另一种负载姜黄素的聚乳酸载药体系被证实能够加速纤维细胞的迁移,从而加速伤口的愈合[86]。 其他小分子活性成分:其他小分子活性成分如一氧化氮[87],糖尿病条件下伤口常用的二甲双胍[88],控制伤口炎症的布洛芬[89]、芝麻酚等[90],在促进伤口愈合的静电纺丝纤维膜制作中也均有运用。现将文中出现的小分子活性药物总结在表1中。 "
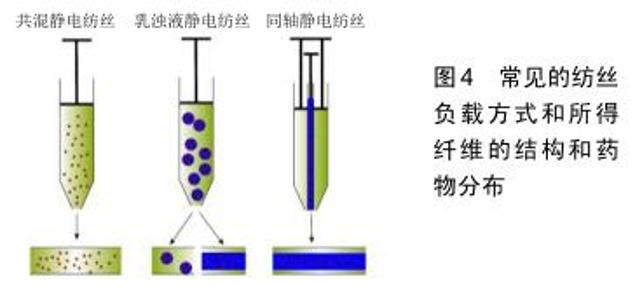
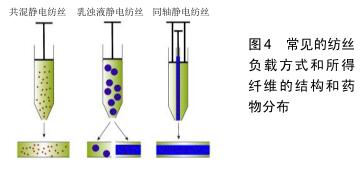
2.3.4 金属活性成分 金属元素在众多生理化学反应中都发挥着重要的作用。相较于前3种活性成分,金属或金属离子的粒径更小,成分也更为单一可控,这是其他活性成分所不具备的。在伤口愈合方面主要是利用金属活性成分的抗菌和调节细胞反应过程的作用,其中最常见的是银元素。 银:银和银离子具有广谱的抗菌作用,被广泛用于伤口愈合的临床治疗[11]。其中纳米银因其大比表面积和易于负载的性质,在促软组织生长的电纺丝膜构建中已得到了较为普遍的运用。纳米银的组装方式包括一体负载和二次装载两种方法:GHAYMINEJAD等[91]报告了通过新型邻苯二酚部分氧化还原化学的制备手段,一体负载并合成了银纳米粒子功能化的电纺纳米纤维,并且证实了其抗菌有效性。而二次装载则是指是先合成纳米银,随后将具有悬浮纳米银的有机溶液进行纺丝[36]。虽然合成方法很多,但是各种负载纳米银的纤维载药体系的抗菌实验结果是相似的,其抗菌效果具有浓度依赖性[36,91]。考虑到细胞毒性的问题,纳米银加载也并不是越多越好,而是需要在抗菌和毒性之间权衡寻找到最佳的含量范围。研究表明,人皮肤成纤维细胞只有在含适量纳米银基质中培养时才能显示出正常的形态和增殖能力[92]。银抗菌的作用机制通常被认为是改变细菌细胞形态和结构,破坏细菌细胞壁;或者直接作用于细菌的DNA,使其失去复制能力[10]。 其他金属成分:除了最常见的银元素外,许多研究者也尝试使用另一些金属成分作为促伤口软组织生长的活性成分。比如负载纳米金和纳米氧化锌静电纺丝载药系统,在相关体内外实验中都观察到了对伤口愈合明显的促进作用的[93-94]。 2.3.5 其他天然成分 上文已经介绍了常见的几大类促伤口软组织愈合活性成分,此外,有许多天然动植物的直接提取物也具抗菌、稳定胶原、改善细胞外基质环境、促进生长因子分泌中的一种或多种特性,所以该研究领域的文献中也有将这些天然成分中作为活性成分实验的报道,例如洋甘菊提取物[95]、金雀异黄素及芦荟提取物等[96-97]。 2.4 构建策略 2.4.1 基础纤维的选择策略 静电纺丝伤口修复载药体系基础的选择个性化程度极高。通常为研究者根据材料制备条件、材料物理性能及聚合物自身的特殊作用综合考虑,综合发挥纤维的优点,单用或者联用多种纤维进行制备。例如使用聚乙烯醇与壳聚糖联用改善可纺性能[22];利用胶原或透明质酸可模拟天然状态下的细胞外基质形态进行伤口修复[19,29];或根据降解时间和强度的不同需求将调节聚乳酸-乙醇酸内组分的比例等[49]。除此之外,抗菌[24]、止血[25]、吸水等一些聚合物本身具有的生物特性也是选择基础纤维时的考虑因素之一[45]。 2.4.2 活性成分的选择策略 静电纺丝伤口修复载药体系的活性成分选择十分灵活多样,应首先考虑伤口类型和相关的基础疾病[10,98],再根据活性成分的作用机制进行匹配,解决最关键的治疗诉求。后文将从临床工作中常面临的伤口处理场景进行活性成分选择策略的介绍。 糖尿病患伤口:罹患糖尿病时,高血糖环境会严重妨碍患者体内的血管新生,造成患者各组织的血液供应受到负面影响。一旦糖尿病患者受到创伤,羸弱的血运重建将会极大地延迟伤口愈合,甚至产生溃烂或坏疽的风险,因此采用促进血管生成对糖尿病患者的伤口处理十分关键[81,99]。从促进血供再生的目的出发,大分子活性物质中的血管内皮生长因子作为公认的血管促进剂可以作为备选[74],能够促进多种生长因子释放的富血小板血浆亦是可选项之一[69]。而对于小分子活性物质,模拟微缺氧应答的二甲基草酰甘氨酸和NO能够促进糖尿病患者伤口的血管再生[81,87],另一方面,含有全反式维甲酸(维生素A类)的静电纺丝伤口修复材料也被报道具有良好的促血管化伤口愈合作用。值得注意的是,除了改善血供的作用靶向之外,使用降糖药降低区域血糖来恢复正常生理过程似乎也是可用方案之一[88]。 感染病患伤口:细菌感染是威胁伤口愈合的最常见因素之一。感染的发生不仅使伤口愈合缓慢,还存在造成菌血症的风险,甚至威胁患者的生命[49]。因此对于有高感染风险的伤口,需要静电纺丝纤维膜具有一定的抗菌性能,以保障组织的正常恢复。最为常见的抗菌活性成分为抗生素类药物,如前文所述的环丙沙星[77]、米诺环素等都已经被用于制备静电纺丝伤口修复载药体 系[78],并且表现出可接受的预后效果。除此之外,具有广谱抗菌性的纳米银、氧化铁和氧化锌等也是被广泛运用的抗菌活性成分[36,93-94];由于这些金属及金属氧化物可能存在无差别的生物毒性,在构建载药体系时具体的负载量需要特别注意[92]。另外,作为生物来源的蛋白类抗菌活性成分,溶菌酶作用更为温和并具有有效的抗菌性能,也可作为活性成分用于对抗伤口感染[75]。 烧伤伤口:烧伤造成的伤口可能伴随着广泛的创面、大量的渗出和沉重的愈合负担,这要求相关的伤口修复材料在具有良好亲水通气功能的基础上,能发挥较强的促进表皮组织再生的能力[29]。自体干细胞活性修复是最为直接有效的构建方案[66],但较大的制备和使用难度使其难以批量生产,运用受限。富血小板血浆和表皮生长因子可加速促进纤维结缔组织、表皮组织的重 建[69-70];其他的天然成分,例如姜黄素也被证实是加速组织再生的有效活性分子[86],以上活性成分均可作为促进烧伤类伤口愈合的选择。 复杂伤口:值得注意的是,伤口难以愈合的风险常由混杂因素导致,临床处理中并不能过于孤立地看待伤口愈合的需求。例如大面积烧伤同样伴随着极高的感染风险[100],联用促表皮生产活性成分和抗菌成分可能是更加有效的解决策略;而糖尿病患者同样也可能存在烧伤和感染伤口的情况,因此需要根据具体情况综合各类伤口的处理思路,共同构建一个有效的活性成分选择策略。 2.5 构建方法 2.5.1 共混静电纺丝 共混纺丝是最为普遍直接的纺丝手段,直接将药物和聚合物混合均匀用于纺丝。由于使用该纺丝方法制备的核心要求在于2种组分能够共同溶解于同种溶剂,例如将脂溶性的药物他扎罗汀和聚己内酯共同溶解于有机溶剂进行纺丝[39],或者是将水溶性的聚乙烯醇、壳聚糖和溶菌酶共同溶解于水中用于材料制备[76]。因此在运用共混静电纺丝时,需要纤维基础和活性成分之间满足共同溶解的要求。另外,考虑到共混静电纺丝中所载药物的突释风险[101],应有效考虑毒性阈值高且在体液环境中较为稳定的活性成分,避免选择脆弱的蛋白(如生长因子)等成分。 2.5.2 乳浊液静电纺丝 针对纤维聚合物和活性成分难以共同溶解的情况,可利用乳浊液纺丝进行制备:将水相和有机相混合以形成乳液,随后将其纺丝得到壳-核结构或分散负载的滴状结构[102]。利用该静电纺丝技术能将水溶性的活性成分例如维生素C,维生素D3和胰岛素等与聚乳酸-乙醇酸共用,制备成为复合要求的静电纺丝伤口屏障[50]。然而由于仍需要将水相和有机相相互混合,乳浊液静电纺丝仍存在使活性蛋白表型而失活的可能[103]。 2.5.3 同轴静电纺丝 当活性分子是脆弱的生长因子或其他大分子蛋白质时,可以采用同轴纺丝直接生成壳-核结构的方法。同轴纺丝运用同心圆状的针头结构,能够同时喷射出2种不同组分的纺丝液,防止成分之间的共混干扰[104]。例如为了封装容易失活的鱼肌浆蛋白及二甲双胍,SENA等[88]研究者就利用了同轴静电纺丝将活性成分与水溶性的聚乙烯醇混合作为核心包封在聚乳酸的外壳内,保护蛋白和药物活性的同时又能够保证成分的缓释,最终显示出促进伤口愈合的潜力。虽然对活性成分的具有良好保护效果,同轴纺丝要求2种原料液体必须在纺丝过程中同步固化[104],这使得这种纺丝方式的技术敏感性较高,可能需要多次尝试和调整纺丝参数。 2.5.4 其他负载方式 除上述3种常见的纺丝方法之外(图4),通过静电吸附或其他化学交联后期负载的方法对于一些药物也是可行的,比如纳米银的负载就最常见的采用后期沉积或吸附方法[36]。 "
|
[1] GURTNER GC, WERNER S, BARRANDON Y, et al. Wound repair and regeneration. Nature. 2008;453(7193):314-321.
[2] BOATENG J, CATANZANO O. Advanced Therapeutic Dressings for Effective Wound Healing--A Review. J Pharm Sci. 2015;104(11): 3653-3680.
[3] WYNN TA, VANNELLA KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis.Immunity. 2016;44(3):450-462.
[4] XUE M, JACKSON CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring.Adv Wound Care (New Rochelle).2015;4(3):119-136.
[5] THU HE, ZULFAKAR MH, NG SF. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing.Int J Pharm. 2012;434(1-2):375-383.
[6] DUMVILLE JC, MCFARLANE E, EDWARDS P, et al. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2015;(4):CD003949.
[7] HYLDIG N, BIRKE-SORENSEN H, KRUSE M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg.2016;103(5):477-486.
[8] DE OLIVEIRA S, ROSOWSKI EE, HUTTENLOCHER A. Neutrophil migration in infection and wound repair: going forward in reverse.Nat Rev Immunol.2016;16(6):378-391.
[9] LIU M, DUAN XP, LI YM, et al. Electrospun nanofibers for wound healing. Mater Sci Eng C Mater Biol Appl.2017;76:1413-1423.
[10] SIMOES D, MIGUEL SP, RIBEIRO MP, et al. Recent advances on antimicrobial wound dressing: A review. Eur J Pharm Biopharm. 2018;127:130-141.
[11] NOROUZI M, BOROUJENI SM, OMIDVARKORDSHOULI N, et al. Advances in Skin Regeneration: Application of Electrospun Scaffolds. Adv Healthc Mater.2015;4(8):1114-1133.
[12] XUE J, XIE J, LIU W, et al. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc Chem Res.2017;50(8):1976-1987.
[13] CHENG F, GAO J, WANG L, et al. Composite chitosan/poly(ethylene oxide) electrospun nanofibrous mats as novel wound dressing matrixes for the controlled release of drugs.J Appl Polym Sci. 2015; 132(24).DOI: 10.1002/app.42060
[14] MIGUEL SP, FIGUEIRA DR, SIMOES D, et al. Electrospun polymeric nanofibres as wound dressings: A review.Colloids Surf B Biointerfaces. 2018;169:60-71.
[15] SOARES RMD, SIQUEIRA NM, PRABHAKARAM MP, et al. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development.Mater Sci Eng C Mater Biol Appl.2018; 92:969-982.
[16] GOMES SR, RODRIGUES G, MARTINS GG, et al.In vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: A comparative study.Mater Sci Eng C Mater Biol Appl. 2015;46:348-358.
[17] GHOSAL K, MANAKHOV A, ZAJICKOVA L, et al. Structural and Surface Compatibility Study of Modified Electrospun Poly(epsilon-caprolactone) (PCL) Composites for Skin Tissue Engineering. AAPS PharmSciTech.2017;18(1):72-81.
[18] LAW JX, LIAU LL, SAIM A, et al. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng Regen Med. 2017;14(6):699-718.
[19] LUO X, GUO Z, HE P, et al. Study on structure, mechanical property and cell cytocompatibility of electrospun collagen nanofibers crosslinked by common agents.Int J Biol Macromol.2018;113:476-486.
[20] EHTERAMI A, SALEHI M, FARZAMFAR S, et al. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromol.2018;117:601-609.
[21] YOUNES I, RINAUDO M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar Drugs. 2015;13(3):1133-1174.
[22] YANG S, LEI P, SHAN Y, et al. Preparation and characterization of antibacterial electrospun chitosan/poly (vinyl alcohol)/graphene oxide composite nanofibrous membrane. Appl Surf Sci. 2018;435:832-840.
[23] ARDESHIRZADEH B, ANARAKI NA, IRANI M, et al. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds.Mater Sci Eng C Mater Biol Appl. 2015;48:384-390.
[24] IGNATOVA M, MANOLOVA N, MARKOVA N, et al. Electrospun Non-Woven Nanofibrous Hybrid Mats Based on Chitosan and PLA for Wound-Dressing Applications.Macromol Biosci. 2009;9(1):102-111.
[25] SANANDIYA ND, LEE S, RHO S, et al. Tunichrome-inspired pyrogallol functionalized chitosan for tissue adhesion and hemostasis.Carbohydr Polym.2019;208:77-85.
[26] CHANDA A, ADHIKARI J, GHOSH A, et al. Electrospun chitosan/ polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications.Int J Biol Macromol. 2018;116: 774-785.
[27] FIGUEIRA DR, MIGUEL SP, DE SA KD, et al. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int J Biol Macromol.2016;93:1100-1110.
[28] 蒋丹,黄建文,邵惠丽,等.丝素蛋白/膀胱脱细胞基质/透明质酸复合纤维支架的制备及生物学性能研究[J].功能材料,2017,48(6):6124-6128.
[29] EBRAHIMI-HOSSEINZADEH B, PEDRAM M, HATAMIAN-ZARMI A, et al. In vivo Evaluation of Gelatin/Hyaluronic Acid Nanofiber as Burn-wound Healing and Its Comparison with ChitoHeal Gel. Fiber Polym.2016;17(6):820-826.
[30] ADUBA DC, YANG H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering (Basel). 2017;4(1).pii: E1..
[31] SAMADIAN H, SALEHI M, FARZAMFAR S, et al. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif Cells Nanomed Biotechnol.2018;46:S964-S974.
[32] LALANI R, LIU L. Electrospun Zwitterionic Poly(Sulfobetaine Methacrylate) for Nonadherent, Superabsorbent, and Antimicrobial Wound Dressing Applications.Biomacromolecules.2012;13(6): 1853-1863.
[33] KUMAR NS, SANTHOSH C, SUDAKARAN SV, et al. Electrospun polyurethane and soy protein nanofibres for wound dressing applications.Iet Nanobiotechnol.2018;12(2):94-98.
[34] ALHUSEIN N, BLAGBROUGH IS, BEETON ML, et al. Electrospun Zein/PCL Fibrous Matrices Release Tetracycline in a Controlled Manner, Killing Staphylococcus aureus Both in Biofilms and Ex Vivo on Pig Skin, and are Compatible with Human Skin Cells. Pharm Res. 2016;33(1):237-246.
[35] ZHANG M, LI X, LI S, et al. Electrospun poly(l-lactide)/zein nanofiber mats loaded with Rana chensinensis skin peptides for wound dressing.J Mater Sci Mater Med. 2016;27(9):136.
[36] WANG Y, LI P, XIANG P, et al. Electrospun polyurethane/keratin/AgNP biocomposite mats for biocompatible and antibacterial wound dressings.J MaterChem B.2016;4(4):635-648.
[37] JU HW, LEE OJ, LEE JM, et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int J Biol Macromol.2016;85:29-39.
[38] SUN HF, MEI L, SONG CX, et al. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials.2006;27(9):1735-1740.
[39] ZHU Z, LIU Y, XUE Y, et al. Tazarotene Released from Aligned Electrospun Membrane Facilitates Cutaneous Wound Healing by Promoting Angiogenesis. ACS Appl Mater Interfaces. 2019;11(39):36141-36153.
[40] MALIKMAMMADOV E, TANIR TE, KIZILTAY A, et al. PCL and PCL-based materials in biomedical applications.J Biomater Sci Polym Ed.2018;29(7-9):863-893.
[41] BAKER SR, BANERJEE S, BONIN K, et al. Determining the mechanical properties of electrospun poly-epsilon-caprolactone (PCL) nanofibers using AFM and a novel fiber anchoring technique. Mater Sci Eng C Mater Biol Appl.2016;59:203-212.
[42] RATHER HA, THAKORE R, SINGH R, et al. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact Mater. 2018;3(2):201-211.
[43] LI X, KANJWAL MA, LIN L, et al. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin.Colloids Surf B Biointerfaces.2013;103:182-188.
[44] PELIPENKO J, KOCBEK P, GOVEDARICA B, et al. The topography of electrospun nanofibers and its impact on the growth and mobility of keratinocytes. Eur J Pharm Biopharm. 2013;84(2):401-411.
[45] ADELI H, KHORASANI MT, PARVAZINIA M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int J Biol Macromol. 2019;122:238-254.
[46] GHALEI S, ASADI H, GHALEI B. Zein nanoparticle-embedded electrospun PVA nanofibers as wound dressing for topical delivery of anti-inflammatory diclofenac. J Appl Polym Sci. 2018;135(33):46643.
[47] RAFIQ M, HUSSAIN T, ABID S, et al. Development of sodium alginate/PVA antibacterial nanofibers by the incorporation of essential oils. Mater Res Express. 2018;5(3).https://doi.org/10.1002/app.46643
[48] ZHAO X, SUN X, YILDIRIMER L, et al. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017;49:66-77.
[49] LIU X, NIELSEN LH, KLODZILISKA SN, et al. Ciprofloxacin-loaded sodium alginate/poly (lactic-co-glycolic acid) electrospun fibrous mats for wound healing.Eur J Pharm Biopharm.2018;123:42-49.
[50] PEH P, LIM NSJ, BLOCKI A, et al. Simultaneous Delivery of Highly Diverse Bioactive Compounds from Blend Electrospun Fibers for Skin Wound Healing. Bioconjug Chem. 2015;26(7):1348-1358.
[51] LI Y, CHEN F, NIE J, et al. Electrospun poly(lactic acid)/chitosan core-shell structure nanofibers from homogeneous solution. Carbohydr Polym. 2012;90(4):1445-1451.
[52] LUO M, MING Y, WANG L, et al. Local delivery of deep marine fungus-derived equisetin from polyvinylpyrrolidone (PVP) nanofibers for anti-MRSA activity. Chem Eng J. 2018;350:157-163.
[53] GUO Z, TANG G, ZHOU Y, et al. Fabrication of Sustained-release CA-PU Coaxial Electrospun Fiber Membranes for Plant Grafting Application. Carbohydr Polym. 2017;169:198-205.
[54] MUTLU G, CALAMAK S, ULUBAYRAM K, et al. Curcumin-loaded electrospun PHBV nanofibers as potential wound-dressing material. J Drug Deliv Sci Technol.2018;43:185-193.
[55] BABAEIJANDAGHI F, SHABANI I, SEYEDJAFARI E, et al. Accelerated Epidermal Regeneration and Improved Dermal Reconstruction Achieved by Polyethersulfone Nanofibers.Tissue Eng Part A. 2010;16(11):3527-3536.
[56] MAVER T, KURECIC M, SMRKE DM, et al. Electrospun nanofibrous CMC/PEO as a part of an effective pain-relieving wound dressing. J Sol-Gel SciTechnol. 2016;79(3):475-486.
[57] QU J, ZHAO X, LIANG Y, et al. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J. 2019;362:548-560.
[58] DOS SANTOS DM, LEITE IS, BUKZEM ADL, et al. Nanostructured electrospun nonwovens of poly(epsilon-caprolactone)/quaternized chitosan for potential biomedical applications. Carbohydr Polym. 2018;186:110-121.
[59] GARCIA-ORUE I, GAINZA G, GARCIA-GARCIA P, et al. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications.Int J Pharm. 2019;556:320-329.
[60] ZHENG Z, LIU Y, HUANG W, et al. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif Cells Nanomed Biotechnol.2018;46:493-501.
[61] GROEBER F, HOLEITER M, HAMPEL M, et al. Skin tissue engineering - In vivo and in vitro applications. Adv Drug Deliv Rev. 2011;63(4-5):352-366.
[62] GU J, LIU N, YANG X, et al. Adiposed-derived stem cells seeded on PLCL/P123 eletrospun nanofibrous scaffold enhance wound healing. Biomed Mater. 2014;9(3):035012.
[63] STEFFENS D, LEONARDI D, DA LUZ SOSTER PR, et al. Development of a new nanofiber scaffold for use with stem cells in a third degree burn animal model.Burns.2014;40(8):1650-1660.
[64] FU Y, GUAN J, GUO S, et al. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis.J Transl Med. 2014;12:274.
[65] BAYATI V, ABBASPOUR MR, DEHBASHI FN, et al. A dermal equivalent developed from adipose-derived stem cells and electrospun polycaprolactone matrix: an in vitro and in vivo study. Anat Sci Int. 2017;92(4):509-520.
[66] DATTA S, RAMESHBABU AP, BANKOTI K, et al. Oleoyl-Chitosan-Based Nanofiber Mats Impregnated with Amniotic Membrane Derived Stem Cells for Accelerated Full -Thickness Excisional Wound Healing. Acs Biomater Sci Eng.2017;3(8):1738-1749.
[67] MAHJOUR SB, FU X, YANG X, et al. Rapid creation of skin substitutes from human skin cells and biomimetic nanofibers for acute full-thickness wound repair. Burns. 2015;41(8):1764-1774.
[68] TAKANARI K, HAZHIZUME R, HONG Y, et al. Skeletal muscle derived stem cells microintegrated into a biodegradable elastomer for reconstruction of the abdominal wall. Biomaterials. 2017;113:31-41.
[69] GOLEBIEWSKA EM, POOLE AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153-162.
[70] CHENG G, MA X, LI J, et al. Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. Int J Pharm.2018;547(1-2):656-666.
[71] KIM HS, YOO HS. In vitro and in vivo epidermal growth factor gene therapy for diabetic ulcers with electrospun fibrous meshes. Acta Biomater. 2013;9(7):7371-7380.
[72] GARCIA-ORUE I, GAINZA G, BORJA GUTIERREZ F, et al. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm. 2017;523(2):556-566.
[73] YANG Y, XIA T, ZHI W, et al. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials.2011;32(18):4243-4254.
[74] WANG K, ZHANG Q, ZHAO L, et al. Functional Modification of Electrospun Poly(epsilon-caprolactone) Vascular Grafts with the Fusion Protein VEGF-HGFI Enhanced Vascular Regeneration. Acs Appl Mater Interfaces.2017;9(13):11415-11427.
[75] TONGLAIROUM P, NGAWHIRUNPAT T, ROJANARATA T, et al. Lysozyme-immobilized electrospun PAMA/PVA and PSSA-MA/PVA ion-exchange nanofiber for wound healing. Pharm Dev Technol. 2015;20(8):976-983.
[76] CHARERNSRIWILAIWAT N, OPANASOPIT P, ROJANARATA T, et al. Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int J Pharm.2012;427(2):379-384.
[77] LI H, WILLIAMS GR, WU J, et al. Thermosensitive nanofibers loaded with ciprofloxacin as antibacterial wound dressing materials. Int J Pharm. 2017;517(1-2):135-147.
[78] ALHUSEIN N, DE BANK PA, BLAGBROUGH IS, et al. Killing bacteria within biofilms by sustained release of tetracycline from triple-layered electrospun micro/nanofibre matrices of polycaprolactone and poly (ethylene-co-vinyl acetate).Drug Deliv Transl Res.2013;3(6):531-541.
[79] ZHU T,PARK HC,SON KM,et al.Effects of dimethyloxalylglycine on wound healing of palatal mucosa in a rat model. Bmc Oral Health. 2015;15:60.
[80] ZHANG Q,OH JH,PARK CH,et al.Effects of Dimethyloxalylglycine- Embedded Poly(epsilon-caprolactone) Fiber Meshes on Wound Healing in Diabetic Rats. Acs Appl Mater Interfaces. 2017;9(9):7950-7963.
[81] REN X,HAN Y,WANG J,et al.An aligned porous electrospun fibrous membrane with controlled drug delivery - An efficient strategy to accelerate diabetic wound healing with improved angiogenesis.Acta Biomater.2018;70:140-153.
[82] LATEEF H, ABATAN OI, ASLAM MN, et al.Topical pretreatment of diabetic rats with all-trans retinoic acid improves healing of subsequently induced abrasion wounds. Diabetes. 2005;54(3):855-861.
[83] LI H, WANG M, WILLIAMS GR,et al.Electrospun gelatin nanofibers loaded with vitamins A and E as antibacterial wound dressing materials. RSC Adv.2016;6(55):50267-50277.
[84] SEDGHI R, SHAABANI A.Electrospun biocompatible core/shell polymer-free core structure nanofibers with superior antimicrobial potency against multi drug resistance organisms.Polymer. 2016;101:151-157.
[85] RANJBAR-MOHAMMADI M,RABBANI S,BAHRAMI SH,et al. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(epsilon-caprolactone) electrospun nanofibers. Mater Sci Eng C Mater Biol Appl.2016;69:1183-1191.
[86] PERUMAL G, PAPPURU S, CHAKRABORTY D, et al. Synthesis and characterization of curcumin loaded PLA-Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater Sci Eng C Mater Biol Appl.2017;76:1196-1204.
[87] RAMADASS SK, NAZIR LS, THANGAM R, et al. Type I collagen peptides and nitric oxide releasing electrospun silk fibroin scaffold: A multifunctional approach for the treatment of ischemic chronic wounds. Colloids Surf B Biointerfaces. 2019;175:636-643.
[88] SENA S, SUMEYRA KN, ULKUGUL G, et al.Controlled Release of Metformin Hydrochloride from Core-Shell Nanofibers with Fish Sarcoplasmic Protein.Medicina (Kaunas). 2019;55(10). pii: E682.
[89] MOHITI-ASLI M, SAHA S, MURPHY SV, et al. Ibuprofen loaded PLA nanofibrous scaffolds increase proliferation of human skin cells in vitro and promote healing of full thickness incision wounds in vivo.J Biomed Mater Res B Appl Biomater.2017;105(2):327-339.
[90] 李兴泽.芝麻酚-CA/Zein复合纳米纤维膜的制备及促进伤口愈合功能评价[D].咸阳:西北农林科技大学, 2019.
[91] GHAYAMINEJAD A, UNNITHAN AR, RAMACHANDRA A, et al. Mussel-Inspired Electrospun Nanofibers Functionalized with Size-Controlled Silver Nanoparticles for Wound Dressing Application. ACS Appl Mater Interfaces.2015;7(22):12176-12183.
[92] GUORUI J, PRABHAKARAN MP, NADAPPURAM BP, et al. Electrospun Poly(L-Lactic Acid)-co-Poly(e-Caprolactone) Nanofibres Containing Silver Nanoparticles for Skin-Tissue Engineering.J Biomater Sci Polym Ed.2012;23(18):2337-52.
[93] YANG X, YANG J, WANG L, et al. Pharmaceutical Intermediate- Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. Acs Nano. 2017;11(6):5737-5745.
[94] HU M, LI C, LI X, et al. Zinc oxide/silver bimetallic nanoencapsulated in PVP/PCL nanofibres for improved antibacterial activity.Artif Cells Nanomed Biotechnol.2018;46(6):1248-1257.
[95] TANG Y, ZHOU Y, LAN X, et al. Electrospun Gelatin Nanofibers Encapsulated with Peppermint and Chamomile Essential Oils as Potential Edible Packaging.J Agric Food Chem. 2019;67(8):2227-2234.
[96] IBRAHIM S, SAYED HM, EL-RAFEI AM, et al. Improved genistein loading and release on electrospun chitosan nanofiber blends.J Mol Liq.2016;223:1056-1061.
[97] MIGUEL SP, RIBEIRO MP, COUTINHO P, et al. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications.Polymers (Basel). 2017;9(5).pii: E183.doi:10.3390/polym9050183.
[98] VEITH AP, HENDERSON K, SPENCER A, et al.Therapeutic strategies for enhancing angiogenesis in wound healing.Adv Drug Deliv Rev. 2019;146:97-125.
[99] SORG H, TILKORN DJ, HAGER S, et al. Skin Wound Healing: An Update on the Current Knowledge and Concepts.Eur Surg Res. 2017; 58(1-2):81-94.
[100] MOFAZZAL JAHROMI MA, SAHANDI ZANGABAD P, MOOSAVI BASRI SM, et al. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing.Adv Drug Deliv Rev. 2018;123:33-64.
[101] YU H,CHEN X,CAI J,et al. Dual controlled release nanomicelle-in- nanofiber system for long-term antibacterial medical dressings.J Biomater Sci Polym Ed.2019;30(1):64-76.
[102] NADA AA, ABDELAZEEM RA, ELGHANDOUR AH, et al. Protection of conjugated linoleic acid into hydrophobic/hydrophilic electrospun fibers. J Drug Deliv Sci Technol.2018;44:482-490.
[103] JIANG H, WANG L, ZHU K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents.J Control Release.2014;193:296-303.
[104] ADELI-SARDOU M, YAGHOOBI MM, TORKZADEH-MAHANI M, et al. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration.Int J Biol Macromol. 2019;124: 478-491.
[105] PILEHVAR-SOLTANAHMADI Y, DADASHPOUR M, MOHAJERI A, et al. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings.Mini Rev Med Chem.2018;18(5):414-427. |
| [1] | Li Li, Ma Li. Immobilization of lactase on magnetic chitosan microspheres and its effect on enzymatic properties [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 576-581. |
| [2] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [3] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [4] | Liu Fang, Shan Zhengming, Tang Yulei, Wu Xiaomin, Tian Weiqun. Effects of hemostasis and promoting wound healing of ozone sustained-release hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3445-3449. |
| [5] | Liu Keke, Duan Xin, Ma Xiangrui, Zhang Yuntao. Effect of cinnamaldehyde on osteoblasts in high glucose environment with the electrospinning membrane as a carrier [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3500-3504. |
| [6] | Zhou Anqi, Tang Yufei, Wu Bingfeng, Xiang Lin. Designing of periosteum tissue engineering: combination of generality and individuality [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3551-3557. |
| [7] | Chen Song, He Yuanli, Xie Wenjia, Zhong Linna, Wang Jian. Advantages of calcium phosphate nanoparticles for drug delivery in bone tissue engineering research and application [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3565-3570. |
| [8] | Lang Limin, He Sheng, Jiang Zengyu, Hu Yiyi, Zhang Zhixing, Liang Minqian. Application progress of conductive composite materials in the field of tissue engineering treatment of myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3584-3590. |
| [9] | Chen Jie, Liao Chengcheng, Zhao Hongbo, Zhao Wei, Chen Zhiwei, Wang Yan. Application of tissue engineering urethral stent and its preparation technology in urethral reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3591-3596. |
| [10] | Chen Zhenyu, Zhang Xiaoning, Luo Yuxin, Liang Jianwei, Yan Chi. Evaluation of silk fibroin/curcumin composite film for promoting wound healing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2554-2561. |
| [11] | Gan Zhoujie, Pei Xibo. Enzyme-responsive nanoparticles in tumor therapy: superiority of nanoparticles in accumulation and drug release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2562-2568. |
| [12] | Xie Jian, Su Jiansheng. Advantages and characteristics of electrospun aligned nanofibers as scaffolds for tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2575-2581. |
| [13] | Yang Lin, Shi Jun, Guo Zhonghua, Wang Zhonghan, Liu He, Li Qiuju. Intervertebral disc tissue engineering based on polymer materials: research focus and hot spots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2589-2596. |
| [14] | Ji Qi, Yu Zhengwen, Zhang Jian. Problems and trends of technique and clinical application of metallic biomaterials prepared by three-dimensional printing technology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2597-2604. |
| [15] | Li Yanle, Yue Xiaohua, Nie Zhen, Zhang Junwei, Li Zhaohui, Nie Weizhi, Jiang Hongjiang. Characteristics and application of bioabsorbable materials in orthopedics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2612-2617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||