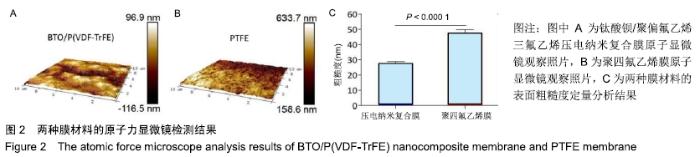
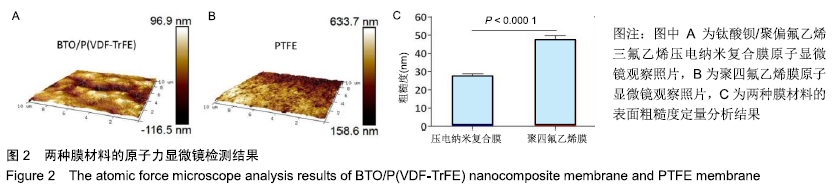
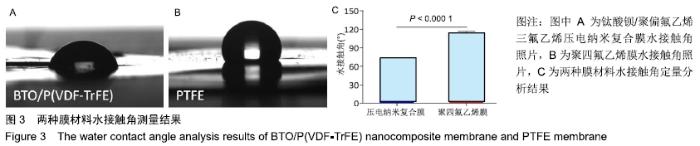
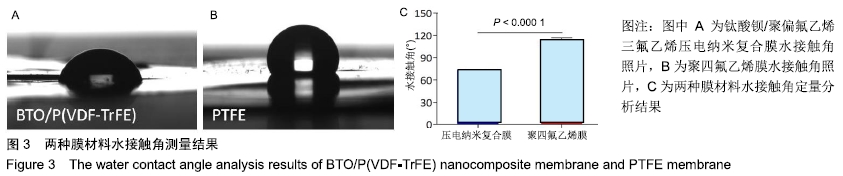
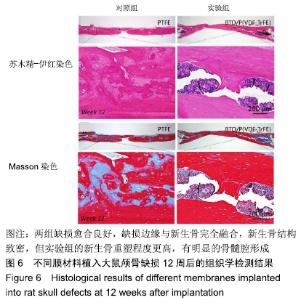
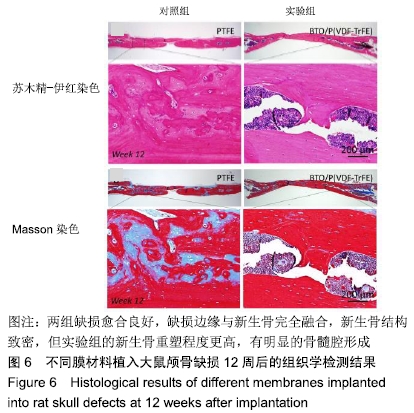
[1] ALGHAMDI AS, CIANCIO SG.Guided tissue regeneration membranes for periodontal regeneration--a literature review.J Int Acad Periodontol.2009;11(3):226-231.
[2] HOORNAERT A, D'ARROS C, HEYMANN MF, et al. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomedl Mater.2016;11(4):045012.
[3] XU C, LEI C, MENG L, et al.Chitosan as a barrier membrane material in periodontal tissue regeneration. J Biomed Mater Res B Appl Biomater.2012;100(5):1435-1443.
[4] MINARY-JOLANDAN M, YU MF.Uncovering nanoscale electromechanical heterogeneity in the subfibrillar structure of collagen fibrils responsible for the piezoelectricity of bone. ACS Nano. 2009;3(7):1859-1863.
[5] HESS R, JAESCHKE A, NEUBERT H, et al.Synergistic effect of defined artificial extracellular matrices and pulsed electric fields on osteogenic differentiation of human MSCs. Biomaterials. 2012;33(35):8975-8985.
[6] JACOB J, MORE N, KALIA K, et al.Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regen.2018;38(1):2.
[7] LOPES HB, FERRAZ EP, ALMEIDA AL, et al.Participation of MicroRNA-34a and RANKL on bone repair induced by poly(vinylidene-trifluoroethylene)/barium titanate membrane.J Biomater Sci Polym Ed.2016;27(13):1369-1379.
[8] FURUYA K, MORITA Y, TANAKA K, et al.Acceleration of osteogenesis by using barium titanate piezoelectric ceramic as an implant material.Proc Spie.2011:7975(19):1779-1781.
[9] CHEN W, YU Z, PANG J, et al.Fabrication of Biocompatible Potassium Sodium Niobate Piezoelectric Ceramic as an Electroactive Implant.Materials.2017;10(4):345.
[10] VANĚK P, KOLSKÁ Z, LUXBACHER T, et al.Electrical activity of ferroelectric biomaterials and its effects on the adhesion, growth and enzymatic activity of human osteoblast-like cells.J Phys D Appl Phys.2016;49(17):175403.
[11] CARVILLE NC, COLLINS L, MANZO M, et al.Biocompatibility of ferroelectric lithium niobate and the influence of polarization charge on osteoblast proliferation and function.J Biomed Mater Res A.2015;103(8):2540-2548.
[12] RIBEIRO C, SENCADAS V, CORREIA DM, et al.Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf B Biointerfaces.2015;136:46-55.
[13] LOPES HB, SANTOS TDS, DE OLIVERIA FS, et al. Poly(vinylidene-trifluoroethylene)/barium titanate composite for in vivo support of bone formation.J Biomater Appl. 2014; 29(1):104-112.
[14] WANG A, LIU Z, HU M, et al.Piezoelectric nanofibrous scaffolds as in vivo energy harvesters for modifying fibroblast alignment and proliferation in wound healing.Nano Energy. 2018;43:63-71.
[15] LI Y, DAI X, BAI Y, et al. Electroactive BaTiO3 nanoparticle- functionalized fibrous scaffolds enhance osteogenic differentiation of mesenchymal stem cells.Int J Nanomedicine. 2017;12:4007-4018.
[16] ZHANG X, ZHANG C, LIN Y, et al.Nanocomposite Membranes Enhance Bone Regeneration Through Restoring Physiological Electric Microenvironment.ACS Nano. 2016; 10(8):7279-7286.
[17] DIBART S.Guided Tissue Regeneration.Practical Periodontal Plastic Surgery.2017:65-68.
[18] ROSALES-LEAL JI, RODRÍGUEZ-VALVERDE MA, MAZZAGLIA G, et al.Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion.Colloid Surface A. 2010; 365(1-3):222-229.
[19] RUPP F, SCHEIDELER L, REHBEIN D, et al.Roughness induced dynamic changes of wettability of acid etched titanium implant modifications.Biomaterials. 2004;25(7): 1429-1438.
[20] LIN D T, YOUNG T H, FANG Y.Studies on the effect of surface properties on the biocompatibility of polyurethane membranes. Biomaterials.2001;22(12):1521-1529.
[21] SHEIKH Z, KHAN AS, ROOHPOUR N, et al.Protein adsorption capability on polyurethane and modified- polyurethane membrane for periodontal guided tissue regeneration applications.Mater Sci Eng C. 2016;68: 267-275.
[22] MCHALE G, SHIRTCLIFFE NJ, NEWTON MI. Super-hydrophobic and super-wetting surfaces:Analytical potential? Analyst.2004;129(4):284-287.
[23] JANSSEN MI, LEEUWEN MB, KOOTEN TG, et al.Promotion of fibroblast activity by coating with hydrophobins in the β -sheet end state.Biomaterials.2004;25(14):2731-2739.
[24] SPICER PP, KRETLOW JD, YOUNG S, et al.Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc.2012;7(10):1918-1929.
[25] FADEL RA,SAMARANI R,CHAKAR C. Guided bone regeneration in calvarial critical size bony defect using a double-layer resorbable collagen membrane covering a xenograft: a histological and histomorphometric study in rats. Oral Maxillofac Surg.2018;22(2):203-213.
[26] NAKAJIMA K, ZHU K, SUN YH, et al.KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis.Nat Commun.2015;6:8532.
[27] NING C, YU P, ZHU Y, et al.Built-in microscale electrostatic fields induced by anatase-rutile-phase transition in selective areas promote osteogenesis.Npg Asia Mater. 2016;8(3): e243.
|