Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (25): 4068-4072.doi: 10.3969/j.issn.2095-4344.2074
Previous Articles Next Articles
Mesenchymal stem cell-derived exosomes in the treatment of orthopedic diseases: roles and prospects
Li Jia1, Wang Zhihui1, Wu Di1, Zhao Yang2
1Department of Joint Surgery, 2Department of Pathology, Affiliated Hospital of Chengde Medical College, Chengde 067000, Hebei Province, China
-
Received:2019-09-07Revised:2019-09-12Accepted:2019-11-07Online:2020-09-08Published:2020-08-26 -
About author:Li Jia, Master, Associate chief physician, Department of Joint Surgery, Affiliated Hospital of Chengde Medical College, Chengde 067000, Hebei Province, China
CLC Number:
Cite this article
Li Jia, Wang Zhihui, Wu Di, Zhao Yang.
Mesenchymal stem cell-derived exosomes in the treatment of orthopedic diseases: roles and prospects [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 4068-4072.
share this article
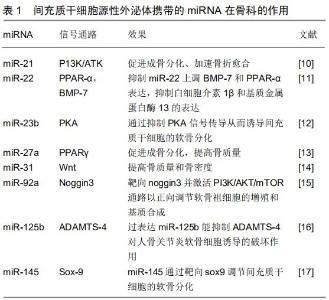
2 结果 Results 2.1 间充质干细胞源性外泌体的功能 外泌体是细胞外囊泡的一种,直径介于30-150 nm之间,形成于内涵体小室,其包含有大量的蛋白分子、DNA、RNA、mRNA 及microRNA等,能够通过细胞间的传递发挥重要作用。外泌体于1983年首先由HARDING等在大鼠的网织红细胞中发现,1987年JOHNSTONE等将这种小囊泡正式定义为“exosomes”,即外泌体。起初外泌体被认为是一种“细胞垃圾”,直到2007年外泌体介导mRNA和miRNA转移才被确定为“细胞间遗传交换的新机制”,并引起人们对外泌体的生物学功能进行了更深入的研究。间充质干细胞广泛存在于人体组织中,同时也是产生外泌体能力最强的细胞。间充质干细胞源性外泌体主要通过收集间充质干细胞上清液来获取,主要提取方法有超速离心法、凝胶色谱法、超滤法、免疫磁珠法和试剂盒提取法。使用超速离心法提取的外泌体纯度较高,是目前外泌体研究的主流方法。间充质干细胞源性外泌体可以通过电子显微镜、纳米粒子跟踪分析等方法直接进行观察。另外,可以利用外泌体的一些表面标志物如CD81、CD9、TSG101、ALIX等,通过微流式细胞分析仪、免疫印迹分析、酶联免疫吸附实验进行检测。脂质和蛋白质是构成外泌体膜的重要组成成分,间充质干细胞源性外泌体不仅表达外泌体特异性表面标志物CD9、CD63、CD81、CD92,还表达间充质干细胞特异性表型CD29、CD44、CD73和CD90等[7],同时携带有HSP8、HSP60、HSP70、HSP9、信号转导蛋白(G蛋白)、多泡体产生相关蛋白(Alix、TSG101)等。此外,外泌体可以携带与其来源细胞相类似的一些细胞因子、脂质、各种mRNA、miRNA、lncRNAs等生物活性物质,在细胞间信息传递过程中发挥重要作用。目前,对间充质干细胞源性外泌体已经鉴定出850种独特的基因产物以及大于150种microRNA[8]。间充质干细胞源性外泌体具有多种与间充质干细胞相似的生物学功能,例如促进细胞迁移、增殖、分化和基质合成等[9]。首先,间充质干细胞源性外泌体表面信号蛋白分子或生物活性脂质配体与靶细胞表面受体相结合,产生信号复合体并激活细胞内信号通路;其次,当组织微环境因疾病或损伤而改变时,通过提供催化活性酶以促进组织稳态,间充质干细胞源性外泌体可以快速恢复正常组织功能;再次,作为间充质干细胞分泌的通信载体,miRNAs的传递可干预靶细胞内目标mRNAs的转录及蛋白质的生成,参与组织修复再生,而miRNA和lncRNA可以激活基因表达的转录调节。除以上的作用机制外,与单独应用间充质干细胞相比,间充质干细胞源性外泌体具有诸多的应用优势,如性质稳定、易于保存、低免疫原性、无细胞活性等诸多优势,为多种疾病的治疗提供了新的手段。间充质干细胞源性外泌体携带的miRNA在骨科的作用,见表1[10-17]。 2.2 间充质干细胞源性外泌体在软骨修复中的作用 骨关节炎是最常见的一种关节疾病,其病理学基础是关节软骨损伤、退变,因此如何最大限度恢复关节软骨的稳态和功能,对于延缓或逆转骨关节炎进展尤为重要。研究表明,软骨下骨缺乏血管、神经组织和致密细胞外基质微环境,阻碍软骨细胞迁移是导致关节软骨难以修复、再生的原因。随着软骨组织工程技术的发展,细胞移植成为治疗软骨损伤的新兴的治疗方式,其中主要包括自体软骨细胞移植和间充质干细胞移植。自体软骨细胞移植技术于1994年由BRITTBERG等[18]首次报道,但目前普遍认为使用自体软骨细胞移植治疗膝关节全层关节软骨缺损的证据不足[19-20]。因此,间充质干细胞源性外泌体移植引起更多的科学关注。 既往理论认为,间充质干细胞的成软骨分化能力和软骨基质分泌能力使其能加快软骨缺损修复[21-25]。最近研究表明间充质干细胞源性外泌体能分泌促进软骨细胞增殖和基质合成的因子,维持微环境的“稳态”,有效促进软骨修复和再生,并已在动物研究以及最近的人体临床试验得到证实[26-27]。WANG等[28]研究表明关节腔内注射间充质干细胞源性外泌体能减轻小鼠半月板切除骨性关节炎模型的软骨细胞破坏和细胞外基质降解。ZHANG等[29]通过建立大鼠骨软骨缺损模型证实间充质干细胞源性外泌体可以促进软骨细胞增殖、迁移和基质合成并减少软骨细胞凋亡。COSENZA等[30]证实小鼠骨髓间充质干细胞源性外泌体通过重建软骨细胞稳态保护软骨细胞免于凋亡,以及刺激巨噬细胞极化达到抗炎作用。骨髓、滑膜、脂肪等不同干细胞源性外泌体对软骨修复有类似的作用[31]。ZHU等[32]比较多能干细胞和滑膜干细胞源性外泌体在胶原酶诱导的小鼠骨关节炎模型中的作用,结果证明二者均可预防骨关节炎的进展。上述研究证实了骨髓间充质干细胞源性外泌体在软骨修复中的作用,但其促进软骨再生的机制尚未完全阐明,一般认为骨髓间充质干细胞源性外泌体富含的多种酶类具有在生物能量代谢、细胞数量和免疫调节等方面恢复细胞内稳态的潜能。ZHANG等[33]研究表明骨髓间充质干细胞源性外泌体介导的软骨修复过程中的细胞快速增殖和浸润归因于CD73介导的AKT和ERK信号传导。此外,干细胞源性外泌体携带遗传物质也能调控软骨细胞修复,例如干细胞源性外泌体中的miR-23b、miR-92a促进软骨细胞增殖[34];miR-125b和miR-320则在软骨细胞基质合成中发挥作用[35];过表达 miR-140-5p的滑膜间充质干细胞来源外泌体可增强软骨组织再生[36]。 2.3 间充质干细胞源性外泌体对椎间盘退变及脊髓损伤的作用 目前认为椎间盘退变机制是髓核细胞数量减少、活性降低,导致细胞外基质合成减少,炎性因子积累,进而出现髓核脱水变性[37]。目前的治疗方法只能不同程度缓解症状,但对于椎间盘退变尚无有效治疗措施,因此如何寻找种子细胞以维持椎间盘形态和功能、逆转椎间盘退行性变成为该领域研究重点。蓝蔚仁等[38]研究表明大鼠髓核细胞外泌体可在体外诱导骨髓间充质干细胞向髓核样细胞分化,且诱导效果优于与髓核细胞的非接触式共培养,可为椎间盘组织工程提供一种有效的髓核细胞来源。蒋长青等[39]在一项体外试验中证实大鼠间充质干细胞源性外泌体能被退变髓核细胞摄取,对退变髓核细胞产生正向营养作用,并且外泌体能改善退变髓核细胞标志基因及蛋白表达。此外,间充质干细胞源性外泌体通过抗氧化和抗炎作用改善椎间盘退变及携带miRNA-21抑制髓核细胞凋亡的作用也得到证实[40-41]。 间充质干细胞源性外泌体不仅可为髓核细胞退变类疾病的治疗提供一种新的思路,还是治疗脊髓损伤最有希望的方法之一。间充质干细胞对组织损伤的修复及再生作用,更大程度上依赖于其对组织内部微环境和细胞功能的调节,但是由于局部缺血坏死、胶质细胞活化、炎症细胞浸润,移植到损伤部位的间充质干细胞仅有少部分存活。目前更多研究倾向于骨髓间充质干细胞的外分泌功能,尤其是外泌体。相对于骨髓间充质干细胞,外泌体含有更多的生长因子(转化生长因子β、成纤维细胞生长因子、血小板源生长因子等和抗炎因子等),更稳定和更容易通过血脊髓屏障发挥作用。王琳等[42-43]将骨髓间充质干细胞源性外泌体作用于脊髓损伤大鼠,结果表明间充质干细胞分泌的外泌体可减少脊髓损伤区域反应性星形胶质细胞的数量,减少神经元的死亡,有利于后肢运动功能改善。周燕等[44]研究结果显示骨髓间充质干细胞外泌体能减轻脊髓组织炎症细胞浸润和髓鞘脱失,减少脊髓A1型星形胶质细胞的活化,促进脊髓损伤后运动功能恢复。尽管外泌体对脊髓损伤的作用得到初步证实,但具体有效成分以及参与调控分化的信号通路尚未清楚.仍需进一步研究。 此外,间充质干细胞源性外泌体在外周神经损伤修复中的作用也得到了证实,MA等[45]研究表明间充质干细胞源性外泌体能成功改善坐骨神经横断损伤大鼠的运动功能、促进轴突和许旺细胞的再生,并减少失神经肌肉萎缩,其主要通过下调促炎细胞因子(白细胞介素6、白细胞介素1β)并增加抗炎细胞因子(白细胞介素10)的表达来促进神经再生。SHIUE等[46]研究表明间充质干细胞源性外泌体能够抑制脊髓神经结扎疼痛诱导的神经炎症并促进抗炎细胞因子和神经营养因子的表达。 2.4 间充质干细胞源性外泌体对骨折愈合及骨代谢的影响 众所周知,正常健康的骨组织在轻度损伤后具有自发再生的能力,然而由疾病或创伤等造成的骨愈合不良、骨缺损修复常需要借助于骨组织工程。使用间充质干细胞移植来促进骨折愈合、骨再生目前已经在临床开始应用,但是间充质干细胞有创性获取、移植细胞活性差和功能障碍的问题仍无法解决。间充质干细胞源性外泌体作为一种无细胞疗法,比活体细胞移植具有明显优势。最新研究表明,各种干细胞源性外泌体都可以发挥调节骨重建、促进体外和体内成骨的作用,这包括骨髓、滑膜、脂肪等来源间充质干细胞等,其中又以骨髓间充质干细胞研究最为深入和广泛。FURUTA等[10]研究发现间充质干细胞源性外泌体能有效加速小鼠股骨骨折修复。QI等[47]利用干细胞来源外泌体修复骨质疏松兔模型骨缺损,证明外泌体能够有效刺激骨髓间充质干细胞的增殖和成骨分化,且该作用随着外泌体浓度增加而增强。朱梦茹等[48]研究表明成骨预处理的脂肪干细胞来源外泌体能够在体外诱导原始脂肪干细胞成骨分化。 目前认为,间充质干细胞源性外泌体在促骨折愈合过程中同时发挥几种作用:①通过自身携带的遗传物质如微小RNA(miRNA)和mRNA等,传递遗传信息调控骨折愈合过程;②通过释放细胞因子和诱导功能性蛋白帮助骨愈合;③通过介导特殊信号通路与转录因子促进骨折愈合。此外,干细胞源性外泌体在调控骨量代谢方面也发挥重要作用。GUO等[49]使用人滑膜衍生间充质干细胞源性外泌体早期静脉治疗大鼠激素性股骨头坏死,结果发现其能够改变股骨头坏死形态,延缓股骨头坏死进程。LIU等[50]研究表明间充质干细胞源性外泌体在大鼠激素性股骨头坏死病变中能抑制骨丢失、改善血管生成,并能激活PI3K/Akt信号通路激发成骨分化。总之,间充质干细胞源性外泌体可通过调控多能干细胞成骨分化,促进新生血管生成,提高无机盐沉积和基质矿化等途径促进骨折愈合,是潜在的促骨折愈合生物学材料。 "
| [1] MAUMUS M, MANFERDINI C, TOUPET K, et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013;11(2):834-844. [2] DORRONSORO A, ROBBINS PD. Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther. 2013;4(2):39. [3] 陈露,孙东.间充质干细胞外泌体在慢性肾脏病的研究进展[J].中华肾脏病杂志,2019,35(3):236-240. [4] 张万松,杨诚,郭文彬,等.BMSCs来源的外泌体对大鼠睾丸缺血再灌注损伤的保护作用[J].南方医科大学学报,2018,38(8):910-916. [5] FAN Y, HERR F, VERNOCHET A, et al. Human Fetal Liver Mesenchymal Stem Cell-Derived Exosomes Impair Natural Killer Cell Function. Stem Cells Dev. 2019;28(1):44-55. [6] TAN CY, LAI RC, WONG W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5(3):76. [7] PASHOUTAN SARVAR D, SHAMSASENJAN K, AKBARZADEHLALEH P. Mesenchymal Stem Cell-Derived Exosomes: New Opportunity in Cell-Free Therapy. Adv Pharm Bull. 2016;6(3):293-299. [8] LAI RC, TAN SS, TEH BJ, et al. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics. 2012;2012:971907. [9] BENTLEY G, BIANT LC, CARRINGTON RW, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230. [10] FURUTA T, MIYAKI S, ISHITOBI H, et al. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med. 2016;5(12):1620-1630. [11] ILIOPOULOS D, MALIZOS KN, OIKONOMOU P, et al. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3(11):e3740. [12] HAM O, SONG BW, LEE SY, et al. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling. Biomaterials. 2012;33(18):4500-4507. [13] QIN Y, WANG L, GAO Z, et al. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961. [14] WANG X, OMAR O, VAZIRISANI F, et al. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS One. 2018;13(2):e0193059. [15] HOU C, ZHANG Z, ZHANG Z, et al. Presence and function of microRNA-92a in chondrogenic ATDC5 and adipose-derived mesenchymal stem cells. Mol Med Rep. 2015;12(4):4877-4886. [16] MATSUKAWA T, SAKAI T, YONEZAWA T, et al. MicroRNA-125b regulates the expression of aggrecanase-1 (ADAMTS-4) in human osteoarthritic chondrocytes. Arthritis Res Ther. 2013;15(1):R28. [17] YANG B, GUO H, ZHANG Y, et al. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS One. 2011;6(7):e21679. [18] BRITTBERG M, LINDAHL A, NILSSON A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-895. [19] VASILIADIS HS, WASIAK J. Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev. 2010;(10):CD003323. [20] LEVINE DW, ROAF PL, DUGUAY SJ. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2009;37(3):e3. [21] VASILIADIS HS, WASIAK J, SALANTI G. Autologous chondrocyte implantation for the treatment of cartilage lesions of the knee: a systematic review of randomized studies. Knee Surg Sports Traumatol Arthrosc. 2010;18(12):1645-1655. [22] LEE KB, HUI JH, SONG IC, et al. Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem Cells. 2007; 25(11):2964-2971. [23] FU WL, ZHOU CY, YU JK. A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med. 2014;42(3):592-601. [24] XIE X, WANG Y, ZHAO C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33(29):7008-7018. [25] CHIANG ER, MA HL, WANG JP, et al. Allogeneic Mesenchymal Stem Cells in Combination with Hyaluronic Acid for the Treatment of Osteoarthritis in Rabbits. PLoS One. 2016;11(2):e0149835. [26] WU L, LEIJTEN JC, GEORGI N, et al. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17(9-10):1425-1436. [27] LAI JH, KAJIYAMA G, SMITH RL, et al. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. [28] WANG Y, YU D, LIU Z, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(1):189. [29] ZHANG S, CHU WC, LAI RC, et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135-2140. [30] COSENZA S, TOUPET K, MAUMUS M, et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8(5): 1399-1410. [31] WU L, PRINS HJ, HELDER MN, et al. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012;18(15-16): 1542-1551. [32] ZHU Y, WANG Y, ZHAO B, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1):64. [33] ZHANG S, CHUAH SJ, LAI RC, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. [34] NING G, LIU X, DAI M, et al. MicroRNA-92a upholds Bmp signaling by targeting noggin3 during pharyngeal cartilage formation. Dev Cell. 2013;24(3):283-295. [35] MENG F, ZHANG Z, CHEN W, et al. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1β-induced chondrocyte responses. Osteoarthritis Cartilage. 2016;24(5):932-941. [36] TAO SC, YUAN T, ZHANG YL, et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180-195. [37] BENDTSEN M, BUNGER C, COLOMBIER P, et al. Biological challenges for regeneration of the degenerated disc using cellular therapies. Acta Orthop. 2016;87(sup363):39-46. [38] 蓝蔚仁,潘赛,孙超,等.大鼠髓核细胞来源外泌体对骨髓间充质干细胞向髓核样细胞分化的作用研究[J].中国脊柱脊髓杂志, 2019,29(1):74-81. [39] 蒋长青,蓝蔚仁,李海音,等.大鼠骨髓间充质干细胞来源外泌体对退变髓核细胞的影响[J].中国脊柱脊髓杂志, 2019,29(2):147-155. [40] CHENG X, ZHANG G, ZHANG L, et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22(1):261-276. [41] XIA C, ZENG Z, FANG B, et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019;143:1-15. [42] 王琳,裴双,郭斌,等.骨髓间充质干细胞来源的外泌体用于大鼠脊髓损伤修复的初步探索[J].中国病理生理杂志, 2018,34(5):862-869. [43] 王琳.骨髓间充质干细胞来源的外泌体对大鼠脊髓损伤后运动功能恢复和星形胶质细胞活化的影响[D]. 郑州:郑州大学, 2018. [44] 周燕,王琳,裴双,等.骨髓间充质干细胞外泌体可减少脊髓损伤后A1型星形胶质细胞的活化[J].中国组织工程研究, 2019,23(21):3294-3301. [45] MA Y, DONG L, ZHOU D, et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med. 2019;23(4): 2822-2835. [46] SHIUE SJ, RAU RH, SHIUE HS, et al. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain. 2019;160(1):210-223. [47] QI X, ZHANG J, YUAN H, et al. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int J Biol Sci. 2016;12(7):836-849. [48] 朱梦茹,郭澍,刘洋,等.脂肪来源干细胞外泌体在组织修复和再生医学中的研究进展[J].中国美容整形外科杂志, 2018,29(12):757-760. [49] GUO SC, TAO SC, YIN WJ, et al. Exosomes from Human Synovial-Derived Mesenchymal Stem Cells Prevent Glucocorticoid- Induced Osteonecrosis of the Femoral Head in the Rat. Int J Biol Sci. 2016;12(10):1262-1272. [50] LIU X, LI Q, NIU X, et al. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int J Biol Sci. 2017;13(2):232-244. |
| [1] | Xu Hui, Kang Bingxin, Zhong Sheng, Gao Chenxin, Zhao Chi, Qiu Guowei, Sun Songtao, Xie Jun, Xiao Lianbo, Shi Qi. Pressing local acupoints plus adjustion of the knee joint in a sitting position for treating knee osteoarthritis: a randomized controlled trial [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 216-221. |
| [2] | Geng Bin, Xia Yayi. Involvement of ERK5 signaling pathway in osteoporosis development in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 178-185. |
| [3] | Li Xiaoqun, Xu Kaihang, Ji Fang. Corylin inhibits osteoclastogenesis and attenuates postmenopausal osteoporosis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 186-190. |
| [4] | Wang Yue, Wang Xinjun, Yuan Yinpeng, Wang Yuze. Mechanism of DAIa2GIP inhibiting mitochondrial apoptosis in chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(11): 1652-1657. |
| [5] | Jia Wei, Zhang Mandong, Chen Weiyi, Wang Chenyan, Guo Yuan. Effects of femoral prosthetic materials on artificial knee arthroplasty performance [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1477-1481. |
| [6] | Fan Haixia, Tan Qingkun, Wang Hong, Cheng Huanzhi, Liu Xue, Ching-chang Ko, Geng Haixia. Rabbit skull defects repaired by the hydroxyapatite/geltin scaffold combined with bone marrow mesenchymal stem cells and umbilical vein endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1495-1499. |
| [7] | Wang Qian, Li Lu, Shu Jingyuan, Dong Zhiheng, Jin Youshi, Wang Qingshan. Micro-morphology and phase of zirconia-based nano-hydroxyapatite functional gradient biomaterials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1517-1521. |
| [8] | Li Rui, Wang Chen, Zhang Wenyi, Ma Shiqing, Sun Yingchun. Various surface treatment methods and resin cement type on the influence of bonding strength of zirconia ceramics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1528-1532. |
| [9] | Xu Changkui, Pu Xiaobing, Lu Yao, Chen Jiarong, Pan Lei. Safety and antibacterial properties of gentamicin-loaded silk fibroin in meniscus repair [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1545-1549. |
| [10] | Zhang Bin, Sun Lihua, Zhang Junhua, Liu Tongbin, Liu Yusan, Cui Caiyun, Li Jun. Short-term effect comparison of a modified socket shield technique and conventional flapless immediate implant and immediate restoration in maxillary aesthetic area [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5514-5519. |
| [11] | Zhou Pengfei, Lin Jing, Chen Yuying, Lin Minkui. Canine dental pulp stem cells-polyglycolic acid scaffold complex for canine periodontal tissue defect [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5526-5531. |
| [12] | Liu Xin, Du Bin, Sun Guangquan, Cao Jinxing, Jiang Xiaohong. Porous beta-tricalcium phosphate-polypyrrole-biotin-icariin composite scaffold promotes recruitment of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5532-5537. |
| [13] | Jiang Zongrui, Zhang Zhiqi. Treatment of meniscus injury or degeneration: the effect and function of stem cells and artificial polymer scaffolds to form tissue engineering system [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5421-5427. |
| [14] | Liao Jian, Huang Xiaolin, Huo Hua, Zhou Qian, Cheng Yuting, Qi Yuhan, Wu Chao, Yang Tongjing, Liao Yunmao, Liang Xing. Effects of calcined bone/chitosan composite materials on proliferation and adhesion in osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5447-5453. |
| [15] | Dong Wenjie, Zhang Shiyang, Zhao Lei, Wang Yukun. Icariin deproteinized inorganic bovine bone composite and deproteinized inorganic bovine bone material in repairing mandibular defects [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5467-5472. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||