Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (25): 4075-4081.doi: 10.3969/j.issn.2095-4344.2017.25.023
Previous Articles Next Articles
Application of human acelluar amniotic membrane in tissue engineered scaffold construction
Yang Ji-bin, Xiong Hua-zhang, Li Yu-wan, Liu Yi
- The First Department of Orthopedics, the Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, Chin
-
Revised:2017-07-03Online:2017-09-08Published:2017-10-09 -
Contact:Liu Yi, Professor, Master’s supervisor, the First Department of Orthopedics, the Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Yang Ji-bin, Studying for master’s degree, the First Department of Orthopedics, the Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:the Science & Technology Program of Guizhou Province, No. SY[2010]3091; the Science & Technology Program of Guizhou Province, No. LH[2016]7477
CLC Number:
Cite this article
Yang Ji-bin, Xiong Hua-zhang, Li Yu-wan, Liu Yi. Application of human acelluar amniotic membrane in tissue engineered scaffold construction[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(25): 4075-4081.
share this article
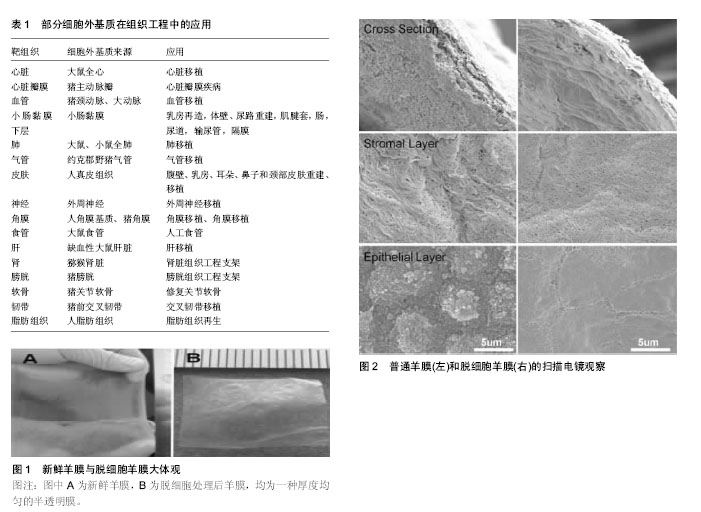
2.1 组织工程概述 近年来,组织工程学在损伤修复、移植物重建和构建新型生物学组织方面已拥有巨大的收获和成果,同时也在生物医学中成为较为活跃的研究方向之一。种子细胞、支架材料及生长因子是组织工程的3大基本要素。鉴于当前不同疾病的在治疗方式上所遇到的难题,组织工程通过种子细胞、支架材料与生长因子的紧密联系,已发展成为治疗疾病和损伤的一种具有广泛前景的方法[16-17]。种子细胞的筛选是构建组织工程的首要环节,来源于不同组织的间充质干细胞是当前构建组织工程种子细胞的最主要来源之一[18]。在组织修复过程中,生长因子一般可通过调节细胞增殖、炎症反应及细胞分化等方式来增强细胞效力及活性;同时,对于细胞间的信号传导,细胞内基因的上调或下调,蛋白的生成,细胞增殖、趋化、血管化等,细胞因子都贯穿其中。目前常见的生长因子,如胰岛素样生长因子、碱性成纤维生长因子、转化生长因子β、血小板源性生长因子、血管内皮生长因子等[19-22]。 应用于组织工程中的支架材料是指具备支撑特定细胞,引导组织再生和控制组织结构,能植入生物体且与组织活体细胞结合的材料[23]。支架材料在组织工程中扮演着极其重要的角色,它可调控细胞在支架中的生长及发挥生物学功能,同时促进搭载在支架中的细胞向目标组织或器官分化[24]。支架为接种的细胞提供初步支撑,将细胞定位在合适的空间中,为细胞的黏附、迁移、增殖和分化提供物理和生物学提示,并繁殖细胞和将其分泌的细胞外基质组装到功能性组织和器官中。在组织再生期间,支架逐渐被细胞自身及周围组织分泌的基质溶解取代。因此,支架应具备良好的生物相容性,可自然降解,并为种子细胞提供合适的信号传递来编排上述生物过程。对支架材料的设计和选择是构建组织工程中急需解决的问题[25]。组织工程支架的类型各异,不同组织所需支架类型也不同,主要包括生物型支架、可降解复合材料支架及天然材料支架。细胞外基质支架属于天然材料型支架,是一种理想的组织工程支架,由于基质蛋白是体内细胞的主要成分[26]。细胞外基质支架不仅对细胞起到支撑作用,同时也对细胞生长、增殖、体态及分化起到调控作用[27]。此外,细胞外基质支架可调节由各种生物活性分子如生长因子和细胞因子活化的信号转导[28-29]。天然细胞外基质由多种蛋白质组成,具有非常复杂的蛋白空间结构。目前许多研究人员一直试图对体内细胞外基质蛋白质进行分离和鉴定[30],但仍有许多蛋白的空间结构尚未明了。因此,通过常规的理化方法难以重构具有与体内细胞外基质相同组成的支架或底物。除了模拟细胞外基质组合物的困难之外,还难以模拟体内细胞外基质的复杂超微结构。对于上述问题,研究人员试图使用经脱细胞化处理的组织和器官的细胞外基质。 2.2 不同组织来源的细胞外基质支架及其相关应用 目前许多研究表明,细胞外基质支架和细胞外基质仿生支架可促进动物和人体许多不同组织的重塑。为了获得天然的细胞外基质仿生支架,常常将组织中的细胞通过脱细胞处理而去除,但其自身结构、功能蛋白质和糖胺聚糖的复杂基质却被可保留下来。现在已有来自各种组织的脱细胞支架,应用于不同形式的组织工程,见表1。这些组织包括心脏和心脏瓣膜[31-35]、血管[36-37]、小肠黏膜下层[38-39]、肺[40-42]、气管[43]、皮肤[44]、神经[45]、角膜[46-47]、食管[48]、肝[49]、肾[50]、膀胱[50-51]、软骨[50,52]、韧带[53]、脂肪组织和人羊膜[54-58]。其中一些已经被商业化用于临床治疗。在市售的细胞外基质支架中,小肠黏膜下层和皮肤衍生的脱细胞支架是最具代表性的支架[39,44]。而人脱细胞羊膜支架因其低免疫原性,容易获得等优势最近被广泛用于不同组织工程的研究。 2.3 脱细胞方法及人脱细胞羊膜的制备 对于细胞外基质支架来说,脱细胞方法的重要性毋庸置疑,目前已有多种脱细胞方法被发明出来[59]。它们大致可分为物理、化学和酶消化法。物理处理通常使用搅动、超声处理、机械按摩、调节压力及反复冻融、以破坏细胞膜并释放细胞内容物。化学处理一般通过使用碱、酸、洗涤剂、有机溶剂、螯合剂、低渗溶液或高渗溶液来破坏细胞膜和负责细胞间和细胞外连接的化学键,达到脱细胞的目的。酶消化法主要使用蛋白酶或核酸酶切割肽键或核苷酸键。所有这些方法都具有各自的优点和局限性。它们可有效去除细胞组分,如细胞核、肌动蛋白和细胞膜,同时它们对剩余细胞外基质的组成、生物活性和生物力学性质也有一些不利影响。通常,将这些方法适当组合以达到最大化脱细胞效应,同时最小化对剩余细胞外基质组成、生物活性、完整性和生物力学性质的任何不利影响。对于特定的组织和器官,应选择适当的方法获得优良的细胞外基质。新鲜人羊膜以及人脱细胞羊膜均为一种厚度均匀的半透明膜,见图1[60]。 人脱细胞羊膜的制备主要包括机械分离、化学、生物制剂单独或联合消化,各种方法均能成功获得人脱细胞羊膜。Wilshaw等[58]在实验中第一次将清洁剂用于脱细胞技术,以0.3 g/L十二烷基硫酸钠、低渗缓冲液和蛋白酶抑制剂、核酸酶、乙二胺四乙酸共同处理羊膜。结果显示:人脱细胞羊膜仍保持良好的生物相容性,细胞呈接触式生长,并且与可溶性组织提取物孵育后,细胞活力未见降低。此外,脱细胞后没有发现极限拉伸强度,延展性或弹性的显著降低,去除细胞成分后无免疫原性,所得到的基质对细胞形态或存活力未表现出不利影响。Li等[63]将新鲜人羊膜修剪后用1% Triton X-100浸泡2 h,洗净后,再予十二烷基硫酸钠于摇床上摇晃12 h,随后PBS洗净。扫描电镜下可见不同层面下细胞被完全移除,见图2。为尽量排除化学试剂对细胞基质可能存在的损伤,Ji等[60]通过反复冻融方法来去除细胞,不仅如此,他们还将羊膜微粒化,最后形成一种具备微载体特征,还完全保留了基底膜结构和丰富的活性物质。Zhang等[61]将冻干人羊膜与0.02%乙二胺四乙酸在37 ℃孵育1 h后,再用细胞刮刀轻柔的刮掉上皮层以达到脱细胞目的。之后通过胶原酶消化不同时间获得不同厚度的人脱细胞羊膜。Gholipourmalekabadi等[55]发明了一种简单、低成本、耗时少的羊膜脱细胞方法,该法使用0.2%乙二胺四乙酸在37 ℃孵育人羊膜30 min,用0.5 mol/L氢氧化钠溶液浸泡30 s后,将其转移到5%氯化铵中剧烈摇晃,可使细胞完全脱落。刘国立等[62]将新鲜人羊膜经SPS溶液漂洗后用戊二醛交联,0.5%十二烷基硫酸钠震荡24 h,胰蛋白酶消化4 h,测定去除细胞成分。"
| [1] Amensag S,McFetridge PS.Rolling the human amnion to engineer laminated vascular tissues.Tissue Eng Part C Methods.2012;18(11):903-912. [2] Buerzle W,Haller CM,Jabareen M,et al.Multiaxial mechanical behavior of human fetal membranes and its relationship to microstructure. Biomech Model Mechanobiol. 2013;12(4): 747-762. [3] Malak TM,Ockleford CD,Bell SC,et al.Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes.Placenta. 1993;14(4):385-406.[4] Riau AK,Beuerman RW,Lim LS,et al.Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010;31(2):216-225.[5] Philips GO,Nather A.The Scientific Basis of Tissue Transplantation. WORLD SCIENTIFIC,2001.[6] Wilshaw SP,Kearney JN,Fisher J,et al.Production of an acellular amniotic membrane matrix for use in tissue engineering.Tissue Eng.2006;12(8):2117-2129.[7] Kheirkhah A,Johnson DA,Paranjpe DR,et al.Temporary sutureless amniotic membrane patch for acute alkaline burns.Arch Ophthalmol.2008;126(8):1059-1066. [8] Maharajan VS,Shanmuganathan V,Currie A,et al.Amniotic membrane transplantation for ocular surface reconstruction: indications and outcomes.Clin Exp Ophthalmol.2007; 35(2): 140-147.[9] Pandey RM,Vajpayee RB,Biswas NR,et al.Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology. 2005;112(11):1963-1969. [10] Jin CZ,Park SR,Choi BH,et al.Human amniotic membrane as a delivery matrix for articular cartilage repair.Tissue Eng. 2007; 13(4):693.[11] Mligiliche N,Endo K,Okamoto K,et al.Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration.J Biomed Mater Res.2002; 63(5):591-600.[12] Mohammad J,Shenaq J,Rabinovsky E,et al.Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg.2000;105(2):660-666.[13] Niknejad H,Peirovi H,Jorjani M,et al.Properties of the amniotic membrane for potential use in tissue engineering.Eur Cell Mater.2008;15(1):88-99.[14] Liliensiek SJ,Nealey P,Murphy CJ.Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering.Tissue Eng Part A. 2009;15(9):2643-2651. [15] Zheng Y,Ji S,Wu H,et al.Topical administration of cryopreserved living micronized amnion accelerates wound healing in diabetic mice by modulating local microenvironment. Biomaterials.2017;113:56-67.[16] Langer R,Vacanti JP.Tissue engineering.Science. 1993; 260(5110):920-926.[17] Griffith LG,aughton G.Tissue Engineering: Current Challenges and Expanding Opportunities. Science. 2002;295(5557): 1009-1014.[18] Cartmell JS,Dunn MG.Development of cell-seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue Eng.2004;10(8):1065-1075.[19] Elisseeff J,McIntosh W,Fu K,et al.Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering.J Orthop Res.2001;19(6):1098-1104.[20] Kanda N, Morimoto N,Ayvazyan AA,et al.Evaluation of a novel collagen-gelatin scaffold for achieving the sustained release of basic fibroblast growth factor in a diabetic mouse model.J Tissue Eng Regen Med.2014;8(1):29-40. [21] Hermanson M,Funa K,Hartman M,et al.Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52(11):3213-3219.[22] Griffith CK,George SC.The effect of hypoxia on in vitro prevascularization of a thick soft tissue. Tissue Eng Part A.2009;15(9):2423-2434.[23] Hoshiba T,Lu H,Kawazoe N,et al.Decellularized matrices for tissue engineering.Expert Opin Biol Ther.2010;10(12):1717- 1728.[24] Badylak SF,Freytes DO,Gilbert TW.Extracellular matrix as a biological scaffold material: Structure and function.Acta Biomater.2009;5(1):1-13.[25] Mano JF,Silva GA,Azevedo HS,et al.Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends.J R Soc Interface. 2007;4(17):999-1030.[26] Adachi E,opkinson I,Hayashi T.Basement-membrane stromal relationships: interactions between collagen fibrils and the lamina densa.Int Rev Cytol.1997;173(173):73.[27] Giancotti FG, Ruoslahti E.Integrin signaling. Science. 1999; 285(5430):1028-1032.[28] Rahman S,Patel Y,Murray J,et al.Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells.BMC Cell Biol.2005; 6(1):8.[29] Comoglio PM,Boccaccio C,Trusolino L.Interactions between growth factor receptors and adhesion molecules: breaking the rules.Curr Opin Cell Biol.2003;15(5):565-671.[30] Manabe R,Tsutsui K,Yamada T,et al.Transcriptome-based systematic identification of extracellular matrix proteins.Proc Natl Acad Sci U S A.2008;105(35):12849-12854.[31] Ott HC,Matthiesen TS,Goh SK,et al.Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med.2008;14(2):213-221.[32] Bertipaglia B,Ortolani F,Petrelli L,et al.Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: the VESALIO Project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur).Ann Thorac Surg.2003;75(4): 1274-1282.[33] Tedder ME,Simionescu A,Chen J,et al.Assembly and testing of stem cell-seeded layered collagen constructs for heart valve tissue engineering.Tissue Eng Part A.2011;17(1-2): 25-36. [34] Schmidt CE,Baier JM.Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials.2000;21(22):2215.[35] Singelyn JM,DeQuach JA,Seif-Naraghi SB,et al.Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering.Biomaterials.2009;30(29):5409.[36] Funamoto S,Nam K, Kimura T,et al.The use of high-hydrostatic pressure treatment to decellularize blood vessels.Biomaterials.2010;31(13):3590-3595.[37] Mcfetridge PS,Daniel JW,Bodamyali T,et al.Preparation of porcine carotid arteries for vascular tissue engineering applications.J Biomed Mater Res A.2004;70A(2):224-234.[38] Badylak SF.The extracellular matrix as a biologic scaffold material.Biomaterials.2007;28(25): 3587.[39] Badylak SF,Freytes DO,Gilbert TW.Extracellular matrix as a biological scaffold material: Structure and function.Acta Biomaterialia.2009;5(1):1-13.[40] Ott HC,Clippinger B,Conrad C,et al.Regeneration and orthotopic transplantation of a bioartificial lung.Nat Med. 2010;16(8):927-933.[41] Petersen TH,Calle EA, Zhao L, et al.Tissue-engineered lungs for in vivo implantation. Science.2010;329(5991):538-541.[42] Price AP,England KA,Matson AM,et al.Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded.Tissue Eng Part A.2010;16(8):2581.[43] Jungebluth P,Go T,Asnaghi A,et al.Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J Thorac Cardiovasc Surg.2009; 138(3): 592-593.[44] Wainwright D,Madden M,Luterman A,et al.Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns.J Burn Care Rehabil.1996;17(2):124-136.[45] Hudson TW,Liu SY,Schmidt CE.Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng.2004;10(10):1346-1358.[46] Choi JS,Williams JK,Greven M,et al.Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials.2010;31(26):6738-6745.[47] Hashimoto Y,Funamoto S,Sasaki S,et al.Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials.2010;31(14):3941-3948.[48] Ozeki M,Narita Y,Kagami H,et al.Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells.J Biomed Mater Res A.2006;79(4):771-778.[49] Uygun BE,Soto-Gutierrez A,Yagi H,et al.Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix.Nat Med.2010;16(7): 814-820.[50] Nakayama KH,Batchelder CA,Lee CI,et al.Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering.Tissue Eng Part A.2010;16(7):2207- 2216.[51] Yang B,Zhang Y,Zhou L,et al.Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering.Tissue Eng Part C Methods.2010;16(5): 1201-1211.[52] Stapleton TW,Ingram J,Katta J,et al.Development and characterization of an acellular porcine medial meniscus for use in tissue engineering.Tissue Eng Part A.2008;14(4):505.[53] Woods T,Gratzer PF.Effectiveness of three extraction techniques in the development of a decellularized bone–anterior cruciate ligament–bone graft. Biomaterials.2005;26(35): 7339.[54] Flynn LE.The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials.2010;31(17): 4715-4724.[55] Gholipourmalekabadi M,Mozafari M,Salehi M,et al.Development of a Cost-Effective and Simple Protocol for Decellularization and Preservation of Human Amniotic Membrane as a Soft Tissue Replacement and Delivery System for Bone Marrow Stromal Cells.Adv Healthc Mater. 2015;4(6):918-926.[56] Chen YJ,Chung MC,Jane Yao CC,et al.The effects of acellular amniotic membrane matrix on osteogenic differentiation and ERK1/2 signaling in human dental apical papilla cells.Biomaterials.2012;33(2):455-463.[57] Minjuan W,Jun X,Shiyun S,et al.Hair Follicle Morphogenesis in the Treatment of Mouse Full-Thickness Skin Defects Using Composite Human Acellular Amniotic Membrane and Adipose Derived Mesenchymal Stem Cells. Stem Cells Int.2016;2016: 8281235.[58] Wilshaw SP,Kearney JN, Fisher J, Iet al.Production of an acellular amniotic membrane matrix for use in tissue engineering.Tissue Eng.2006;12(8):2117-2129.[59] Gilbert TW,Sellaro TL,Badylak SF.Decellularisation of Tissues and Organs.Biomaterials.2006;27(19):3675.[60] Ji SZ,Xiao SC,Luo PF,et al.An epidermal stem cells niche microenvironment created by engineered human amniotic membrane.Biomaterials.2011;32(31):7801-7811.[61] Zhang L,Zou D,Li S,et al.An Ultra-thin Amniotic Membrane as Carrier in Corneal Epithelium Tissue-Engineering.Sci Rep. 2016;6:21021.[62] 刘国立,于昆仑,白江博,等.脱细胞羊膜与医用膜修复腱鞘缺损防治肌腱粘连的比较[J].中国组织工程研究,2016,20(21):3117- 3123.[63] Li W,Ma G,Brazile B,et al.Investigating the Potential of Amnion-Based Scaffolds as a Barrier Membrane for Guided Bone Regeneration.Langmuir.2015;31(31):8642-8653.[64] Akazawa K,Iwasaki K,Nagata M,et al.Double-layered cell transfer technology for bone regeneration.Sci Rep. 2016;6: 33286. [65] Liu PF,Guo L,Zhao DW,et al.Study of human acellular amniotic membrane loading bone marrow mesenchymal stem cells in repair of articular cartilage defect in rabbits.Genet Mol Res.2014;13(3):7992-8001.[66] Chehelcheraghi F,Eimani H,Homayoonsadraie S,et al.Effects of Acellular Amniotic Membrane Matrix and Bone Marrow-Derived Mesenchymal Stem Cells in Improving Random Skin Flap Survival in Rats. Iran Red Crescent Med J.2016;8(6):e25588.[67] Huang G,Ji S,Luo P,et al.Accelerated expansion of epidermal keratinocyte and improved dermal reconstruction achieved by engineered amniotic membrane.Cell Transplant.2013;22(10): 1831-1844.[68] Mahmoudi-Rad M,Abolhasani E,Moravvej H,et al.Acellular amniotic membrane: an appropriate scaffold for fibroblast proliferation.Clin Exp Dermatol.2013;38(6):646-651.[69] Zhang L,Zou D,Li S,et al.An Ultra-thin Amniotic Membrane as Carrier in Corneal Epithelium Tissue-Engineering.Sci Rep. 2016;6:21021.[70] 范巨峰,芮宏亮,宋森,等.人尿道黏膜上皮细胞系与脱细胞羊膜基质复合培养的实验研究[J].中国美容整形外科杂志,2016,27(4): 246-249.[71] 袁丁,罗晗,黄斌,等.人脱细胞羊膜治疗下肢静脉性溃疡的初步报告[J].中国修复重建外科杂志,2014,28(11):1449-1450.[72] Yuan J,Li W,Huang J,et al.Transplantation of human adipose stem cell-derived hepatocyte-like cells with restricted localization to liver using acellular amniotic membrane.Stem Cell Res Ther. 2015;6:217. [73] Kasimir MT,Rieder E,Seebacher G,et al.Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves.J Heart Valve Dis.2006;15(2): 286-290.[74] Badylak SF.The extracellular matrix as a biologic scaffold material.Biomaterials.2007;28(25):3587. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1530-1536. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||