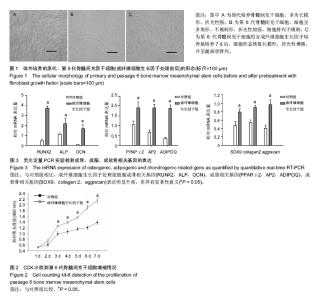
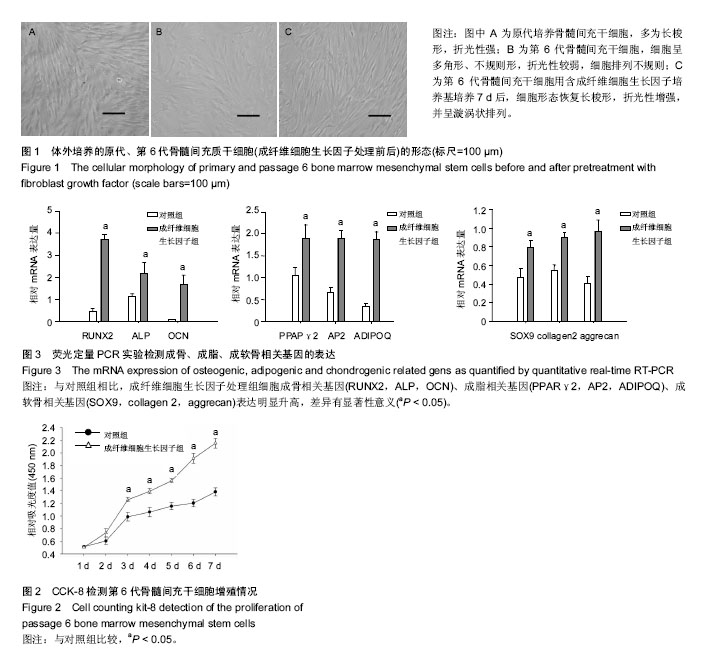
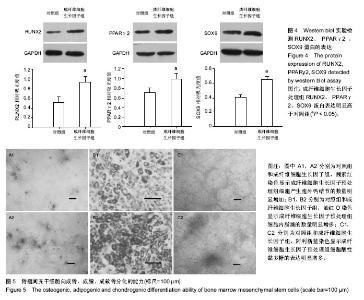
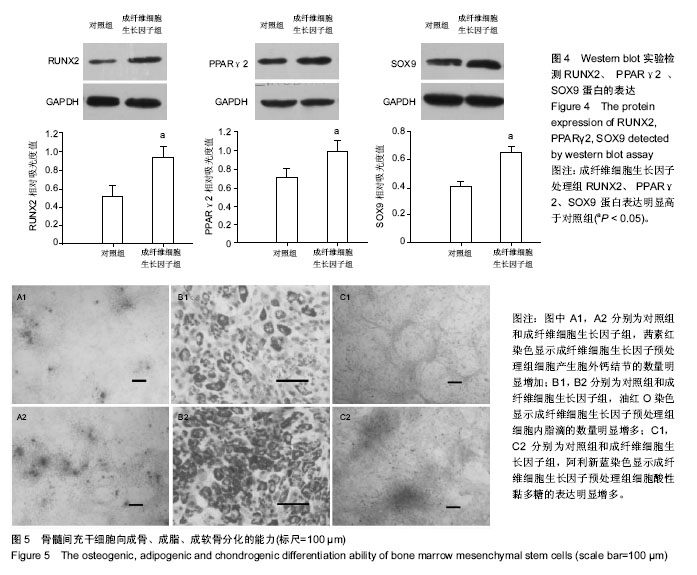
| [1] Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281-294.[2] Comite P, Cobianchi L, Avanzini MA, et al. Isolation and ex vivo expansion of bone marrow-derived porcine mesenchymal stromal cells: potential for application in an experimental model of solid organ transplantation in large animals. Transplant Proc. 2010;42(4):1341-1343.[3] Kim J, Kang JW, Park JH, et al. Biological characterization of long-term cultured human mesenchymal stem cells. Arch Pharm Res. 2009;32(1):117-126.[4] Wagner W, Bork S, Lepperdinger G, et al. How to track cellular aging of mesenchymal stromal cells. Aging (Albany NY). 2010;2(4):224-230.[5] Fickert S, Schröter-Bobsin U, Gross AF, et al. Human mesenchymal stem cell proliferation and osteogenic differentiation during long-term ex vivo cultivation is not age dependent. J Bone Miner Metab. 2011;29(2):224-235.[6] 张明伟,于晓妉,郭子宽,等.体外长期培养对人骨髓间充质干细胞生物学特性的影响[J].组织工程与重建外科杂志,2005,1(1): 51-53.[7] Eom YW, Oh JE, Lee JI, et al. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2014;445(1):16-22.[8] Xu C, Rosler E, Jiang J, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23(3):315-323.[9] Dvorak P, Dvorakova D, Koskova S, et al. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells. 2005;23(8):1200- 1211.[10] 温秀杰,刘鲁川,王尧,等.碱性成纤维生长因子和表皮生长因子对脊髓神经干细胞增殖与分化的影响[J].中国临床神经科学,2014, 22(2):121-125.[11] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.[12] Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414(6859):118-121.[13] Pountos I, Corscadden D, Emery P, et al. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 2007;38 Suppl 4:S23-33.[14] Eom YW, Oh JE, Lee JI, et al. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2014;445(1):16-22.[15] Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16(2):107-137.[16] Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3): 235-253.[17] Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006; 24(11):2412-2419.[18] Lee JH, Um S, Jang JH, et al. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012;348(3):475-484.[19] Bianchi G, Banfi A, Mastrogiacomo M, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287(1):98-105.[20] Martin I, Muraglia A, Campanile G, et al. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138(10):4456-6442.[21] Tsutsumi S, Shimazu A, Miyazaki K, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288(2):413-419.[22] Cheng T, Yang C, Weber N, et al. Fibroblast growth factor 2 enhances the kinetics of mesenchymal stem cell chondrogenesis. Biochem Biophys Res Commun. 2012; 426(4):544-550.[23] 苏钰涵,杜华,牛广明,等.成纤维细胞生长因子的信号通路[J].中国组织工程研究,2016,20(15):2255-2264. |