Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (13): 2023-2028.doi: 10.3969/j.issn.2095-4344.2017.13.010
Previous Articles Next Articles
Effect of cultivation systems on the maintenance of human umbilical cord mesenchymal stem cell characteristics and their proliferation rate in vitro
Sun Ya-ru, Zhang Bing-qiang, Wang Fu-bin, Xu Ping, Wang Er-pu, Li Cui-cui
- Cell Transplantation Laboratory of Ministry of Health, Qingdao Municipal Hospital, Qingdao 266000, Shandong Province, China
-
Revised:2016-12-16Online:2017-05-08Published:2017-06-09 -
Contact:Sun Ya-ru, Cell Transplantation Laboratory of Ministry of Health, Qingdao Municipal Hospital, Qingdao 266000, Shandong Province, China -
About author:Sun Ya-ru, Master, Ministry of Health Cell Transplantation Laboratory, Qingdao Municipal Hospital, Qingdao 266000, Shandong Province, China Zhang Bing-qiang, Master, Cell Transplantation Laboratory of Ministry of Health, Qingdao Municipal Hospital, Qingdao 266000, Shandong Province, China Sun Ya-ru and Zhang Bing-qiang contributed equally to this work.
CLC Number:
Cite this article
Sun Ya-ru, Zhang Bing-qiang, Wang Fu-bin, Xu Ping, Wang Er-pu, Li Cui-cui. Effect of cultivation systems on the maintenance of human umbilical cord mesenchymal stem cell characteristics and their proliferation rate in vitro[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(13): 2023-2028.
share this article
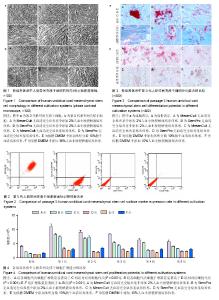
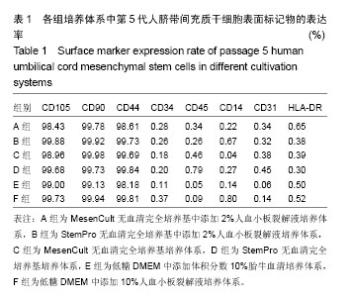
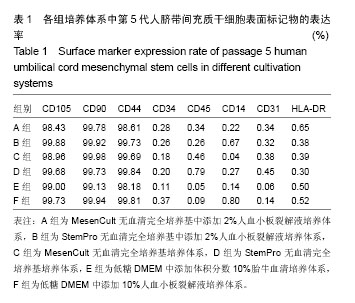
2.1 培养体系对脐带间充质干细胞形态学的影响 D组第3代细胞形态细长、大小不均,E、F组第3代细胞形态扁平,其余组第3代细胞均为长梭形且大小较均一(图1a)。将各组细胞传代至第5代,结果显示A、B组细胞形态及大小较第3代时无明显变化,C组细胞形态较第3代时趋于扁平且大小不均一,D组细胞形态较第3代时细长,E组细胞形态较第3代时扁平,F组细胞形态较第3代时形态无明显变化(图1b)。 2.2 培养体系对脐带间充质干细胞表面标志物表达率的影响 表面标志物流式检测结果显示,各组CD105、CD90、CD44阳性表达率均大于95%,且组间无明显表达差异;CD34、CD45、CD14、CD31和HLA-DR阳性表达率均小于2%,各组间无明显差异(图2,表1)。 2.3 培养体系对脐带间充质干细胞诱导分化能力的影响 第5代细胞成脂诱导结果显示,A、B组成脂诱导率较高,脂滴分布较培养E、F组密集,C、D组诱导率较低(图3a)。第5代细胞成骨诱导结果显示,A、B组钙盐沉淀着色较E、F组深,C、D组钙盐沉淀较少(图3b)。 2.4 培养体系对脐带间充质干细胞扩增效率的影响 A组各细胞代次细胞扩增数量显著高于C相组对应的细胞代次(P < 0.001),B组各细胞代次细胞扩增数量显著高于D组相对应细胞代次(P < 0.001);E、F组扩增数量显著低于A、B组(P < 0.001),见图4。"

| [1] Parekkadan B,Milwid JM.Mesenchymal stem cells as therapeutics.Annu Rev Biomed Eng.2010;12:87-117.[2] Servais S,Beguin Y,Delens L,et al.Novel approaches for preventing acute graft-versus-host disease after allogeneic hematopoieticstem cell transplantation.Expert Opin Investig Drugs.2016;25(8):957-972. [3] Wystrychowski W,Patlolla B,Zhuge Y,et al.Multipotency and cardiomyogenic potential of human adipose-derived stem cells from epicardium, pericardium, and omentum.Stem Cell Res Ther.2016;7(1):84-95. [4] Jiang R,Han Z,Zhuo G,et al.Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study.Front Med.2011;5(1):94-100.[5] Deng P,Torrest A,Pollock K,et al.Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells.Neural Regen Res.2016;11(5):702-705. [6] Labrador S,Alonso ML,Alvarez S,et al.Mesenchymal stem cell therapy in retinal and optic nerve diseases: An update of clinical trials.World J Stem Cells.2016;8(11):376-383.[7] Moussa L,Pattappa G,Doix B, et al.A biomaterial-assisted mesenchymal stromal cell therapy alleviates colonic radiation-induced damage.Biomaterials.2017;115:40-52.[8] Mao X,Liu Y,Chen C,et al.Mesenchymal Stem Cells and Their Role in Dental Medicine. Dent Clin North Am.2017;61(1): 161-172. [9] Cui GH,Wang YY,Li CJ,et al.Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis.Exp Ther Med.2016;12(5):3390-3400. [10] Oner A,Gonen ZB,Sinim N,et al.Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study.Stem Cell Res Ther.2016;7(1):178.[11] Dothel G,Raschi E,Rimondini R,et al.Mesenchymal stromal cell-based therapy: Regulatory and translational aspects in gastroenterology.World J Gastroenterol.2016; 22(41):9057- 9068.[12] Can A,Balci D.Isolation,culture,and characterization of human umbilical cord stroma-derived mesenchymal stem cells. Methods Mol Biol.2011;698:51-62.[13] Gong W, Han Z, Zhao H,et al.Banking human umbilical cord-derived mesenchymal stromal cells for clinical use.Cell Transplant.2012;21(1):207-216.[14] Zhou HX,Liu ZG,Liu XJ,et al.Umbilical cord-derived mesenchymal stem cell transplantation combined with hyperbaric oxygen treatment for repair of traumatic brain injury.Neural Regen Res.2016;11(1): 107-113.[15] Guo ZY,Sun X,Xu XL,et al.Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res. 2015;10(4): 651-658. [16] Zhao Q,Wang XY,Yu XX,et al.Expression of human telomerase reverse transcriptase mediates the senescence of mesenchymal stem cells through the PI3K/AKT signaling pathway.Int J Mol Med.2015;36(3):857-864.[17] Mizuno M,Katano H,Otabe K,et al.Platelet-derived growth factor (PDGF)-AA/AB in human serum are potential indicators of the proliferative capacity of human synovial mesenchymal stem cells.Stem Cell Res Ther.2015;6:243-253.[18] Esmaeli A,Moshrefi M,Shamsara A,et al.Xeno-free culture condition for human bone marrow and umbilical cord matrix-derived mesenchymalstem/stromal cells using human umbilical cord blood serum.Int J Reprod Biomed (Yazd). 2016; 14(9):567-576.[19] Fazzina R,Iudicone P,Fioravanti D,et al.Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues. Stem Cell Res Ther.2016; 7(1):122.[20] Smith JR,Pfeifer K,Petry F,et al.Standardizing Umbilical Cord Mesenchymal Stromal Cells for Translation to Clinical Use: Selection of GMP-Compliant Medium and a Simplified Isolation Method.Stem Cells Int.2016;2016:6810980.[21] Kocaoemer A,Kern S,Klüter H,et al.Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue.Stem Cells. 2007;25(5):1270-1278. [22] Tateishi K,Ando W,Higuchi C,et al.Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: Potential feasibility for clinical applications.Cell Transplant.2008;17(5):549-557.[23] Yilmaz M,Ovali E,Akdogan E,et al.Autologous serum is more effective than fetal bovine serum on proliferation of bone marrow derived human mesenchymal stem cells.Saudi Med J.2008;29(2):306-309.[24] Juhl M,Tratwal J,Follin B,et al.Comparison of clinical grade human platelet lysates for cultivation of mesenchymal stromal cells from bone marrow and adipose tissue.Scand J Clin Lab Invest.2016;76(2):93-104. [25] Stute N,Holtz K,Bubenheim M,et al.Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use.Exp Hematol.2004;32(12):1212-1225.[26] Lange C,Cakiroglu F,Spiess AN,et al.Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine.J Cell Physiol.2007;213(1):18-26.[27] Chase LG,Lakshmipathy U,Solchaga LA,et al.A novel serum-free medium for the expansion of human mesenchymal stem cells.Stem Cell Res Ther.2010;1(1):8.[28] Haack-Sorensen M,Friis T,Bindslev L,et al.Comparison of different culture conditions for human mesenchymal stromal cells for clinical stem cell therapy.Scand J Clin Lab Invest. 2008;68(3):192-203.[29] Merlo B,Pirondi S,Iacono E,et al. Viability, in vitro Differentiation and Molecular characterization of equine adipose tissue-derived mesenchymal stem cells cryopreserved in serum and serum-free medium. Cryo Letters. 02016;37(4):243-252.[30] Zanicotti DG,Coates DE.Growing Adipose-Derived Stem Cells Under Serum-Free Conditions.Methods Mol Biol. 2017; 1537: 439-446.[31] Escobar CH,Chaparro O.Xeno-Free Extraction, Culture, and Cryopreservation of Human Adipose-Derived Mesenchymal Stem Cells.Stem Cells Transl Med.2016; 5(3): 358-365.[32] Wu X,Kang H,Liu X,et al.Serum and xeno-free, chemically defined, no-plate-coating-based culture system for mesenchymal stromal cells from the umbilical cord.Cell Prolif. 2016;49(5):579-88.[33] Wu JY,Lu Y,Chen JS,et al.Pooled Umbilical Cord Blood Plasma for Culturing UCMSC and Ex Vivo Expanding Umbilical Cord Blood CD34? Cells.Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23(4):1112-1119.[34] Chase LG,Yang S,Zachar V,et al.Development and characterization of a clinically compliant xeno-free culture medium in good manufacturing practice for human multipotent mesenchymal stem cells.Stem Cells Transl Med. 2012;1(10):750-758. [35] Simões IN,Boura JS,dos Santos F,et al.Human mesenchymal stem cells from the umbilical cord matrix: successful isolation and ex vivo expansion using serum-/xeno-free culture media. Biotechnol J.2013;8(4):448-458. [36] Swamynathan P,Venugopal P,Kannan S,et al.Are serum-free and xeno-free culture conditions ideal for large scale clinical grade expansion of Wharton's jelly derived mesenchymal stem cells? A comparative study. Stem Cell Res Ther.2014; 5(4):88. [37] Rakian R,Block TJ,Johnson SM,et al.Native extracellular matrix preserves mesenchymal stem cell "stemness" and differentiation potential under serum-free culture conditions. Stem Cell Res Ther.2015;6:235. [38] Gottipamula S,Ashwin KM,Muttigi MS,et al.Isolation, expansion and characterization of bone marrow-derived mesenchymal stromal cells in serum-free conditions.Cell Tissue Res. 2014;356(1):123-135. [39] Mizukami A,Fernandes-Platzgummer A,Carmelo JG,et al.Stirred tank bioreactor culture combined with serum-/xenogeneic-free culture medium enables an efficient expansion of umbilical cord-derived mesenchymal stem/stromal cells.Biotechnol J. 2016;11(8):1048-1059. [40] Gerby S,Attebi E,Vlaski M,et al.A new clinical-scale serum-free xeno-free medium efficient in ex vivo amplification of mesenchymal stromal cells does not support mesenchymal stem cells.Transfusion.2016.doi: 10.1111/trf.13902. [Epub ahead of print] |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [5] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [6] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [7] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [8] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [9] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [10] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [11] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [12] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [13] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [14] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [15] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||