Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (5): 773-779.doi: 10.3969/j.issn.2095-4344.2017.05.020
Previous Articles Next Articles
Anti-oxidative activities of supernatants of human fetal placental mesenchymal stem cells cultured in serum-free medium
Fu Xue1, 2, Zhang Yu-jie1, 2, Yan Xiu-rui2, Ma Xiao-na2, Liu Xiao-ming2, Wei Jun1, 2
- 1Clinical Medicine College, Ningxia Medical University, Yinchuan 750003, Ningxia Hui Autonomous Region, China
2Ningxia Human Stem Cell Institute, Affiliated Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China
-
Online:2017-02-18Published:2017-03-20 -
Contact:Wei Jun, Master, Clinical Medicine College, Ningxia Medical University, Yinchuan 750003, Ningxia Hui Autonomous Region, China; Ningxia Human Stem Cell Institute, Affiliated Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
About author:Fu Xue, Studying for master’s degree, Clinical Medicine College, Ningxia Medical University, Yinchuan 750003, Ningxia Hui Autonomous Region, China; Ningxia Human Stem Cell Institute, Affiliated Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81460247
CLC Number:
Cite this article
Fu Xue, Zhang Yu-jie, Yan Xiu-rui, Ma Xiao-na, Liu Xiao-ming, Wei Jun. Anti-oxidative activities of supernatants of human fetal placental mesenchymal stem cells cultured in serum-free medium[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(5): 773-779.
share this article
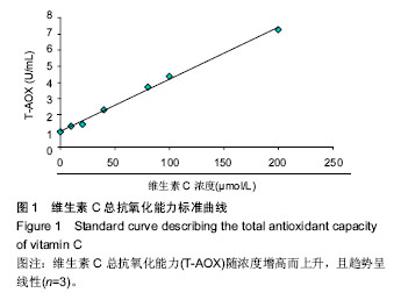
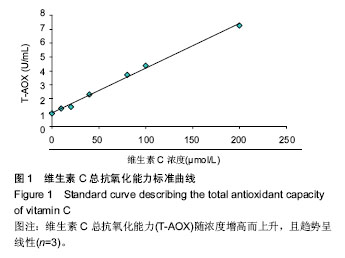
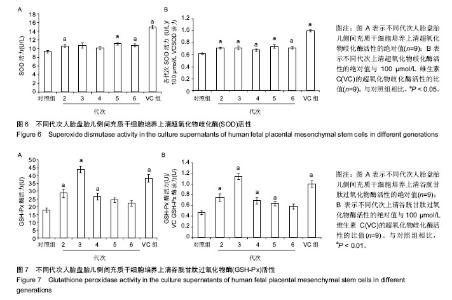
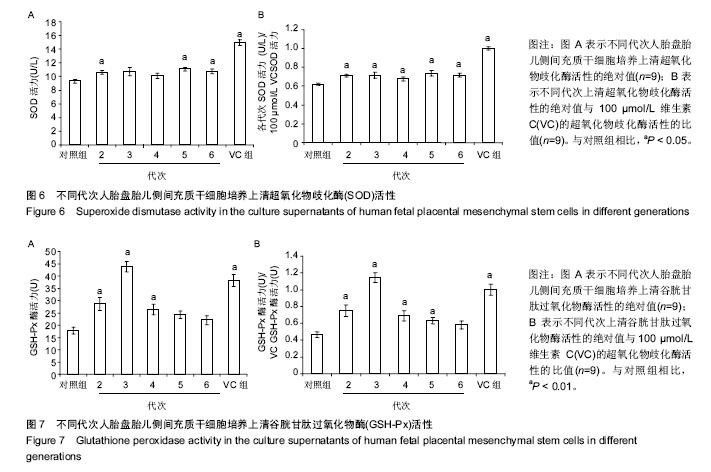
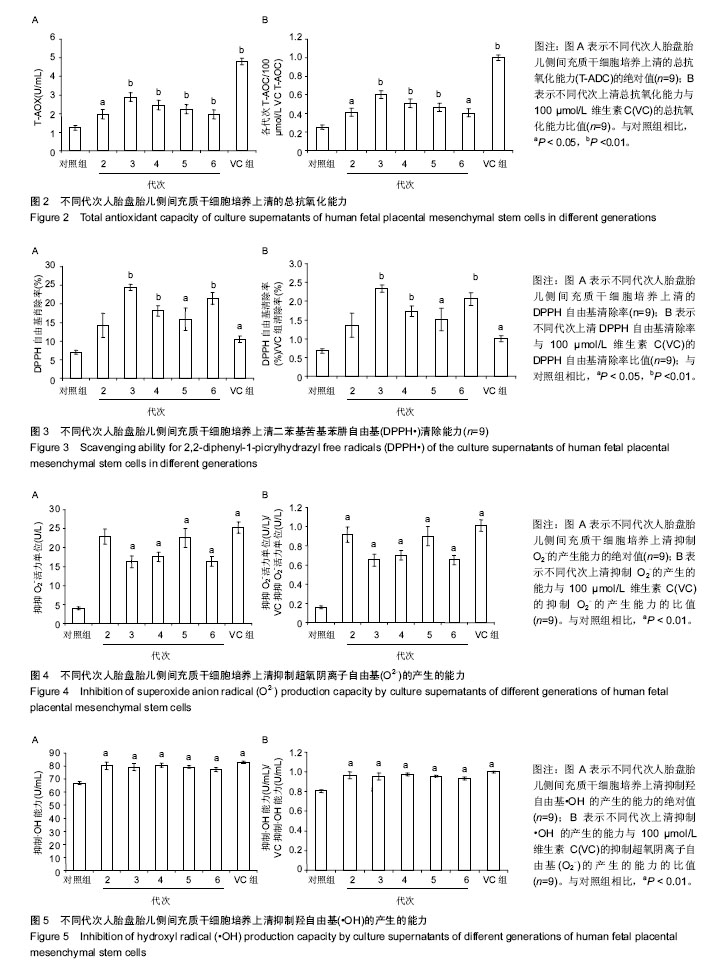
2.2 人胎盘胎儿侧间充质干细胞培养上清的总抗氧化能力 上清中的抗氧化物质可以将Fe3+还原成Fe2+,后者可与菲啉类物质形成稳定的络合物,通过比色法测定出其抗氧化能力的高低。此实验中,各代次上清均具有一定的的总抗氧化能力,且各代次之间的总抗氧化能力存在一定趋势,以P3-P4代总抗氧化能力较高。与空白对照组相比,差异均具有统计学差异(P < 0.01或P < 0.05;图2A)。与维生素C的总抗氧化能力的标准曲线对比可见,人胎盘胎儿侧间充质干细胞培养上清的总抗氧化能力相当于40-80 μmol/L维生素C。 为了消除不同时间测定以及不同试剂盒的组间差异,实验将各代次上清总抗氧化能力的绝对值与同组100 μmol/L维生素C进行对比,求出比值(图2B),而所得结果与使用绝对值的统计结果类似,由此可知实验重复性较好,可信度较高。 2.3 人胎盘胎儿侧间充质干细胞培养上清对DPPH自由基清(DPPH•)除能力 DPPH•是一种以氮为中心的自由基,含有一个单电子,在517 nm处有强烈的吸收峰,其乙醇溶液呈深紫色,加入培养上清后,测定清除DPPH•而引起的吸光度的减少可以反映其抗氧化能力的强弱。实验结果显示不同代次的培养上清均具有一定的清除DPPH·的能力,以P3及P6代清除率较高,且各组清除率绝对数值均超过维生素C组。除P2代外,其清除率与空白对照组相比存在显著差异(P < 0.01或 P;图3A)。通过将人胎盘胎儿侧间充质干细胞培养上清对DPPH•的清除率对比求出比值后发现结果类似(图3B)。 < 0.05 2.4 人胎盘胎儿侧间充质干细胞培养上清抑制O2-的活力单位 此实验模拟机体中黄嘌呤与黄嘌呤氧化酶反应系统,产生超氧阴离子自由基,加入电子传递物质及gress显色剂,使反应体系呈现紫红色,可用分光光度计测其吸光度。实验的结果显示格代次上清中抑制O2-的能力以P2及P5代抑制作用较强,其绝对值与空白对照组相比均存在显著差异(P < 0.01;图4A),且消除组间差异后,结果类似(图4B)。 2.5 人胎盘胎儿侧间充质干细胞培养上清抑制羟自由基 (•OH)活力单位 羟自由基是一种氧化能力很强的自由基,其性质很活泼,氧化各种有机物和无机物的速率极快,是造成组织脂质过氧化、核酸断裂、蛋白质和多糖分解的主要因素,与人体衰老、肿瘤、辐射孙损伤和细胞吞噬能力有关。因此,清除羟自由基对抗氧化具有重要意义。实验显示各代次上清对O2-的均具有抑制作用,与空白对照组相比均差异存在显著性意义(P < 0.01),但从绝对值上看,各代次间无差异(图5A,B)。 2.6 人胎盘胎儿侧间充质干细胞培养上清超氧化物歧化酶活力 超氧化物歧化酶是人体内清除超氧阴离子自由基的酶,在维持机体自由基平衡上有重要作用。实验通过模拟黄嘌呤及黄嘌呤氧化酶系统产生超氧阴离子自由基,后者氧化羟胺形成亚硝酸盐,在显色剂作用下呈现紫红色,用可见光分光光度计测定其吸光度。当被测样本中含超氧化物歧化酶时,则亚硝酸盐减少,比色管吸光度值降低。实验显示,除P3,P4代外各代次上清超氧化物歧化酶活力与空白对照组相比差异有显著性意义(P < 0.05;图6A),但消除了组间差异后,P3,P4代上清超氧化物歧化酶活力与空白对照组相比差异有显著性意义(P < 0.05;图6B)。 2.7 人胎盘胎儿侧间充质干细胞培养上清谷胱甘肽过氧化物酶活力 谷胱甘肽过氧化物酶是人体内广泛存在的一种重要的过氧化物分解的酶,它特异地催化还原型谷胱甘肽对氢过氧化物的还原反应。一般认为它在细胞内能清除有害的过氧化物代谢产物,阻断脂质过氧化链锁反应,从而起到保护细胞膜结构和功能完整的作用。实验显示除P6代外,各代次上清谷胱甘肽过氧化物酶活力与空白对照组相比存在显著差异(P < 0.01;图7A),消除了组间差异后结果也类似(图7B)。"
| [1] 李昂,邢雅琪,李晓霞,等.氧化应激中ROS对FOXO3a转录因子的调控作用研究进展[J].中国药理学通报,2016,32(9):1203-1207.[2] Poljsak B, Suput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013(1):1-11.[3] Ni S, Wang D, Qiu X, et al. Bone marrow mesenchymal stem cells protect against bleomycin-induced pulmonary fibrosis in rat by activating Nrf2 signaling. Int J Clin Exp Pathol. 2015; 8(7):7752-7761.[4] Lin KC, Yip HK, Shao PL, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173- 185.[5] Dey R, Kemp K, Gray E, et al. Human mesenchymal stem cells increase anti-oxidant defences in cells derived from patients with Friedreich's ataxia. Cerebellum. 2012;11(4): 861-871.[6] Liu X, Zhou L, Chen X, et al. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;61:437-448.[7] Park CM, Kim MJ, Kim SM, et al. Umbilical cord mesenchymal stem cell-conditioned media prevent muscle atrophy by suppressing muscle atrophy-related proteins and ROS generation. In vitro cell dev biol Anim. 2016;52(1):68-76.[8] Turkoglu A, Duru ME, Mercan N, et al. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007;101(1):267-273.[9] Zhang Z, Liu X, Zhang X, et al. Comparative evaluation of the antioxidant effects of the natural vitamin C analog 2-O-beta-D-glucopyranosyl-L-ascorbic acid isolated from Goji berry fruit. Arch Pharmacal Res. 2011;34(5):801-810.[10] Costa S, Reina-Couto M, Albino-Teixeira A, et al. Statins and oxidative stress in chronic heart failure. Rev Port Cardiol. 2016;35(1):41-57.[11] Mota S I, Costa R O, Ferreira I L, et al. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer's disease. Biochim Biophys Acta. 2015;1852(7):1428-1441.[12] Aouacheri O, Saka S, Krim M, et al. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Can J Diabetes. 2015;39(1):44-49.[13] Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol Res. 2015; 100: 170-174.[14] 刘冲,彭御冰,王忠.Keap1-Nrf2-ARE信号通路在多器官疾病中的研究进展[J].中国临床医学,2015,22(2):239-243.[15] Cao W, Cao K, Cao J, et al. Mesenchymal stem cells and adaptive immune responses. Immunol Lett. 2015;168(2): 147-153.[16] Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3(3):1-11.[17] Merino-Gonzalez C, Zuniga FA, Escudero C, et al. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol. 2016;7:1-9.[18] Shalaby SM, El-Shal AS, Abd-Allah SH, et al. Mesenchymal stromal cell injection protects against oxidative stress in Escherichia coli-induced acute lung injury in mice. Cytotherapy. 2014;16(6):764-75.[19] 杜莉莉,吕润潇,杨晓漪,等.胎盘间充质干细胞低氧培养液对肠黏膜上皮细胞氧化应激损伤的保护作用[J].中国医科大学学报, 2016, 45(2):131-140.[20] Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837-3844.[21] Martinet L, Fleury-Cappellesso S, Gadelorge M, et al. A regulatory cross-talk between Vgamma9Vdelta2 T lymphocytes and mesenchymal stem cells. Eur J Immunol. 2009;39(3):752-762.[22] Tasso R, Augello A, Carida M, et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30(1): 150-157.[23] Sabin K, Kikyo N. Microvesicles as mediators of tissue regeneration. Transl Res. 2014;163(4):286-295.[24] Sdrimas K, Kourembanas S. MSC microvesicles for the treatment of lung disease: a new paradigm for cell-free therapy. Antioxid Redox Signal. 2014;21(13):1905-1915.[25] Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831-840.[26] Ahmadi M, Rahbarghazi R, Aslani M R, et al. Bone marrow mesenchymal stem cells and their conditioned media could potentially ameliorate ovalbumin-induced asthmatic changes. Biomed Pharmacother. 2016;85:28-40.[27] Chen KH, Chen CH, Wallace CG, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute is. Oncotarget. 2016; 7(46):74537-74556.[28] Toh WS, Lai RC, Hui JH, et al. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2016.[29] Reza AM, Choi YJ, Yasuda H, et al. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 2016;6: 38498.[30] Ti D, Hao H, Fu X, et al. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci China Life Sci. 2016.[31] Gopal SK, Greening DW, Rai A, et al. Extracellular vesicles: their role in cancer biology and epithelial-mesenchymal transition. Biochem J. 2017;474(1):21-45.[32] Quek C, Bellingham SA, Jung CH, et al. Defining the purity of exosomes required for diagnostic profiling of small RNA suitable for biomarker discovery. RNA Biol. 2016.[33] Nuzhat Z, Kinhal V, Sharma S, et al. Tumour-derived exosomes as a signature of pancreatic cancer-liquid biopsies as indicators of tumour progression. Oncotarget. 2016.[34] Foj L, Ferrer F, Serra M, et al. Exosomal and Non-Exosomal Urinary miRNAs in Prostate Cancer Detection and Prognosis. Prostate. 2016.[35] Wu J, Wang Y, Li L. Functional significance of exosomes applied in sepsis: a novel approach to therapy. Biochim Biophys Acta. 2016.[36] Nedaeinia R, Manian M, Jazayeri MH, et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2016.[37] Thulin P, Hornby RJ, Auli M, et al. A longitudinal assessment of miR-122 and GLDH as biomarkers of drug-induced liver injury in the rat. Biomarkers. 2016:1-9. [38] Ming Z, Zhou R, Chen XM. Regulation of Host Epithelial Responses to Cryptosporidium Infection by MicroRNAs. Parasite Immunol. 2016.[39] Tsukita S, Yamada T, Takahashi K, et al. MicroRNAs 106b and 222 Improve Hyperglycemia in a Mouse Model of Insulin-Deficient Diabetes via Pancreatic β-Cell Proliferation. EBioMedicine. 2016.[40] Mirzaei H, Sahebkar A, Jaafari MR, et al. Diagnostic and Therapeutic Potential of Exosomes in Cancer: The Beginning of a New Tale? J Cell Physiol. 2016. |
| [1] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [2] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [3] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [4] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [5] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [6] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [7] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [8] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [9] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [10] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [11] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [12] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [13] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [14] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [15] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||