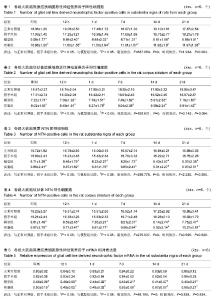
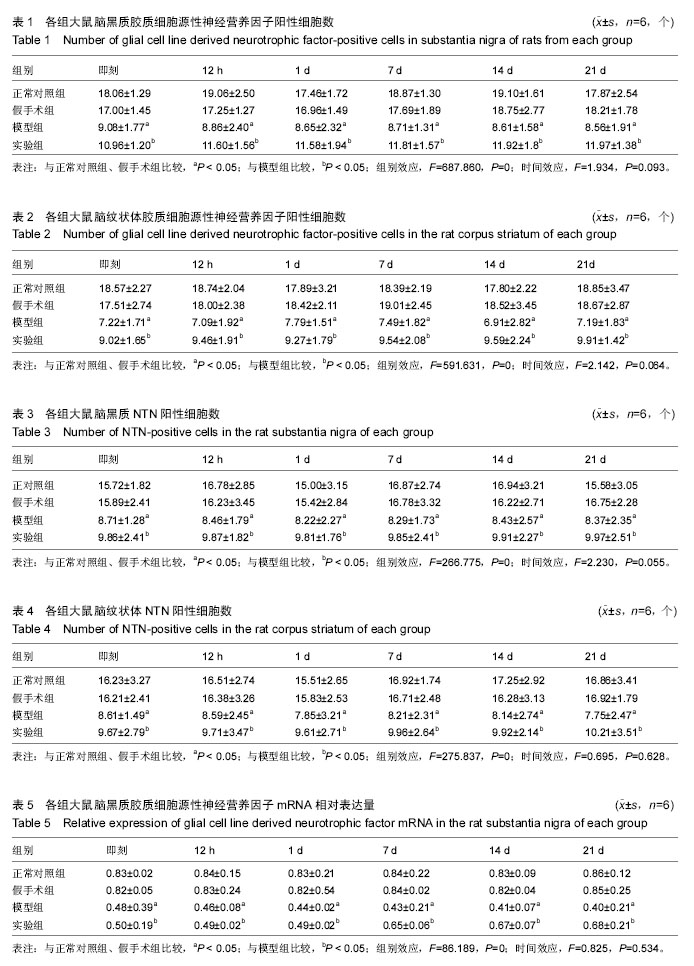
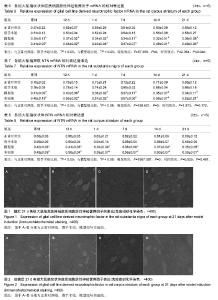
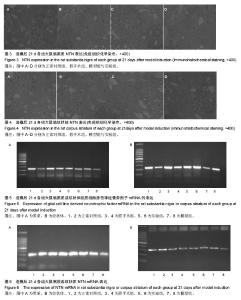
| [1] Wang M,Li F,Shi Z,et al.N-cadherin is a novel ERαanchor that protects against 6-OHDA damage to dopaminergic cells.Cell Mol Neurobiol.2014;34(1):123-131.
[2] Zhang C,Jin Y,Ziemba KS,et al.Long distance directional growth of dopaminergic axons along pathways of netrin-1 and GDNF. Exp Neurol.2013; 250:156-164.
[3] Mickiewicz AL,Kordower JH.GDNF family ligands: a potential future for Parkinson's disease therapy.CNS Neurol Disord Drug Targets.2011;10(6):703-711.
[4] Quintino L,Baudet A,Larsson J,et al.FACS binding assay for analysing GDNF interactions.J Neurosci Methods.2013;218(1):25-28.
[5] Meka DP,Müller-Rischart AK,Nidadavolu P,et al.Parkin cooperates with GDNF/RET signaling to prevent dopaminergic neuron degeneration.J Clin Invest.2015; 125(5):1873-1885.
[6] Yang F,Zhou L,Wang D,et al.Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1αthrough SIRT-3/PHD-2 degradation pathway.Neuroscience.2015;304:250-259.
[7] Soliman S,Ishrat T,Fouda AY,et al.Sequential Therapy with Minocycline and Candesartan Improves Long- Term Recovery After Experimental Stroke.Transl Stroke Res.2015;6(4):309-322.
[8] Yan P,Zhu A,Liao F,et al.Minocycline reduces spontaneous hemorrhage in mouse models of cerebral amyloid angiopathy. Stroke.2015;46(6):1633-4160.
[9] Hou Y,Heon Ryu C,Jun JA,et al.Interferon β-secreting mesenchymal stem cells combined with minocycline attenuate experimental autoimmune encephalomyelitis. J Neuroimmunol.2014;274(1-2):20-27.
[10] Kumar A,Chaudhary T,Mishra J.Minocycline modulates neuroprotective effect of hesperidin against quinolinic acid induced Huntington's disease like symptoms in rats: behavioral, biochemical, cellular and histological evidences.Eur J Pharmacol.2013;720(1-3):16-28.
[11] Ma J,Zhang J,Hou WW,et al.Early treatment of minocycline alleviates white matter and cognitive impairments after chronic cerebral hypoperfusion. Sci Rep.2015;5:12079.
[12] 潘贺,潘丽红,张予阳.米诺环素对脑缺血后的神经保护作用及其机制[J].中国卒中杂志,2011,28(3):1644-1646.
[13] 蔡晶晶,赵平.米诺环素对大鼠视神经损伤后视网膜神经节细胞的保护及对GDNF和VEGF表达的影响[D].河北医科大学,2013.
[14] Stirling DP,Koochesfahani KM,Steeves JD,et al. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11(4):308-322.
[15] 邢红霞,彭海,孙圣刚.同型半胱氨酸对多巴胺能神经元的作用及其机制的研究[J].中国老年学杂志,2004,24(12): 1162-1165.
[16] Rickert U,Grampp S,Wilms H,et al.Glial Cell Line-Derived Neurotrophic Factor Family Members Reduce Microglial Activation via Inhibiting p38MAPKs-Mediated Inflammatory Responses.J Neurodegener Dis.2014;2014:369468.
[17] Mickiewicz AL,Kordower JH.GDNF family ligands: a potential future for Parkinson's disease therapy.CNS Neurol Disord Drug Targets.2011;10(6):703-711.
[18] Hegarty SV,Sullivan AM,O'Keeffe GW.BMP2 and GDF5 induce neuronal differentiation through a Smad dependant pathway in a model of human midbrain dopaminergic neurons.Mol Cell Neurosci.2013;56:263-271.
[19] Zhang C,Jin Y,Ziemba KS,et al.Long distance directional growth of dopaminergic axons along pathways of netrin-1 and GDNF.Exp Neurol.2013;250:156-164.
[20] Kim HS,Suh YH.Minocycline and neurodegenerative diseases.Behav Brain Res.2009; 196(2):168-179.
[21] Zhao C,Ling Z,Newman MB,et al.TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice .Neurobiol Dis. 2007; 26(1): 36-46. |