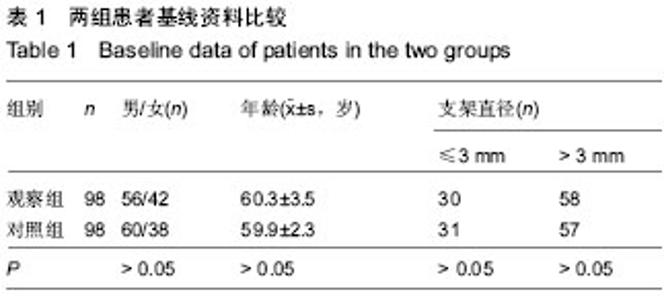
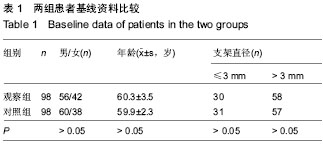
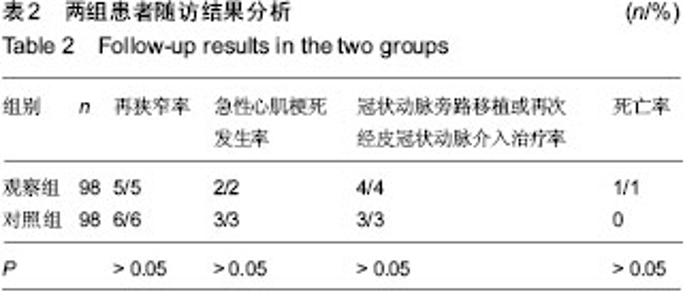
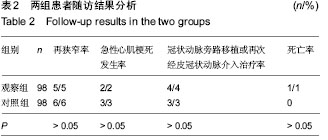
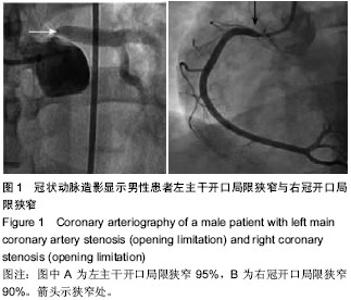
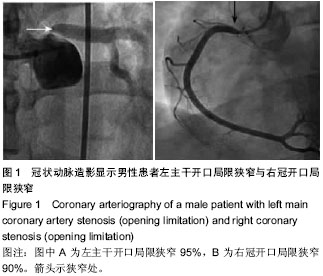
| [1] 王哲颖,刘同库.雷帕霉素洗脱支架治疗无保护冠状动脉左主干严重狭窄性病变的安全性和远期效果研究[J].中国全科医学, 2013,16(27):3192-3195.
[2] 周旭晨,曾定尹,关启刚,等.雷帕霉素与紫杉醇药物洗脱支架对小型猪早期冠状动脉炎症性狭窄治疗的定量冠状动脉造影研究[J].中国循环杂志,2006,21(4):262-265.
[3] 刘强,刘幼文,彭长农,等.雷帕霉素洗脱支架治疗冠状动脉长病变的随访观察[J].中国基层医药,2005,12(6):655-656.
[4] 厉其昀,陈忠,顾兴中,等.一种新型聚合物携带雷帕霉素洗脱血管支架抑制内膜增生的效应[J].中国组织工程研究与临床康复,2009,13(4):626-630.
[5] 吴轶喆.完全可降解聚L-乳酸的生物相容性及其用于冠脉支架的实验研究[D].复旦大学,2012.
[6] 刘爱宁.药物洗脱支架在冠状动脉介入治疗中的应用[J].中国组织工程研究与临床康复,2007,11(25):4981-4984.
[7] 刘斌,李淑梅,张基昌,等.生物可降解高分子材料载内皮前体细胞CD34抗体涂层支架防治犬冠脉再狭窄的研究[J].中国实验诊断学,2007,11(3):369-371.
[8] Serruys PW,Degertekin M,Tanabe K,et al.Intravascular ultrasound findings in the multicenter, randomized, double-blind RAVEL (RAndomized study with the sirolimus-eluting VElocity balloon-expandable stent in the treatment of patients with de novo native coronary artery Lesions) trial.Circulation.2002;106(7):798-803.
[9] Morice MC,Serruys PW,Sousa JE,et al.A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization.N Engl J Med.2002;346(23): 1773-1780.
[10] 徐孝玄,黄怡.QT离散度与冠状动脉狭窄病变的关系及冠状动脉支架置入术对它的影响[J].临床和实验医学杂志,2006,5(11): 1685-1687.
[11] 高成志.药物洗脱支架与裸支架置入术治疗冠状动脉狭窄伴瘤样扩张的长期对比观察[D].山东大学,2010.
[12] 李国华,邢启崇,宁美芳,等.HSRA联合PTCA或支架成形术治疗老年钙化性冠状动脉狭窄[J].中国中西医结合影像学杂志,2004, 2(1):31-33,45.
[13] 胡金朋,浦奎,王津胜,等.药物洗脱支架治疗老年多支冠状动脉狭窄122例[J].中国老年学杂志,2012,32(6):1287-1288.
[14] 潘荣荣.两种涂层基质雷帕霉素支架治疗冠脉弥漫性病变的临床对比研究[D].大连医科大学,2011.
[15] 陈宏.冠心病患者应用可降解涂层雷帕霉素洗脱支架与PARTNER支架的随机对照研究[D].中国医科大学,2009.
[16] Regar E,Serruys PW,Bode C,et al.Angiographic findings of the multicenter Randomized Study With the Sirolimus-Eluting Bx Velocity Balloon-Expandable Stent (RAVEL): sirolimus-eluting stents inhibit restenosis irrespective of the vessel size.Circulation.2002;106(15):1949-1956.
[17] 黄丽霞.冠脉内药物洗脱支架植入术的护理体会[J].右江民族医学院学报,2007,29(1):150-151.
[18] 苗志林.Rho激酶在炎症因子诱导的小型猪冠状动脉狭窄病变的表达及雷帕霉素干预的研究[D].中国医科大学,2005.
[19] 苗志林,曾定尹,孙喜琢,等.白介素-1β诱导小型猪冠状动脉内膜增殖时Rho激酶表达与雷帕霉素干预的研究[J].中华心血管病杂志,2006,34(5):445-449.
[20] 郝六一,程凌倩,张俊霞,等.经桡动脉应用改良Crush技术植入药物洗脱支架治疗冠状动脉分叉病变的疗效分析—附47例报告[J].新医学,2009,40(6):387-389.
[21] 郑勇.药物洗脱支架在糖尿病冠状动脉长病变中的应用研究[J].医学综述,2012,18(21):3682-3683.
[22] 田刚,张春晓,孙中华,等.冠状动脉支架置入术后支架内再狭窄的相关因素分析[J].山东医药,2012,52(34):53-55.
[23] 付鑫,吴桂平,张彦红,等.多层螺旋CT评价靶血管通畅性图像的质量:冠状动脉支架材料及其结构有影响吗?[J].中国组织工程研究与临床康复,2009,13(52):10297-10301.
[24] 杨菲菲,王禹,荆晶,等.无保护左主干病变支架介入治疗后冠状动脉造影随访结果分析[J].中华老年心脑血管病杂志,2010,12(9): 795-798.
[25] 李朝晖,党瑜华,刘新民,等.西罗莫司洗脱支架治疗糖尿病患者冠状动脉狭窄病变的临床研究[J].中国医药指南,2013, 11(12): 646-647.
[26] 付乃宽,曹月娟,吴冬燕,等.药物涂层支架治疗冠状动脉分叉病变临床结果观察[J].中华老年心脑血管病杂志,2008,10(6): 464-464.
[27] 张秋月.冠状动脉狭窄及支架内再狭窄的影像学诊断研究进展[J].医学综述,2014,20(9):1652-1654.
[28] 柴宝红,Lu Qun.用于冠状动脉支架内再狭窄的西罗莫司类药物的研究进展[J].中国抗生素杂志,2008,33(7):392-395,432.
[29] 蒋学祥,邱建星,刘剑,等.64层螺旋CT评估冠状动脉狭窄的准确性—与传统冠状动脉造影对照研究[J].中国医学影像技术,2006, 22(10):1472-1476.
[30] 孙荣跃,沈岚,乔文龙,等.冠状动脉造影及支架植入术在冠状动脉狭窄中的应用[J].上海医学影像,2002,11(4):247-249.
[31] 董何彦.药物涂层冠状动脉金属支架的载药性能[C].//第十一届全国数学药理学学术会议论文集,2007:47-58.
[32] 马生山.冠状动脉狭窄支架置入术前后314例心电图分析[J].中国误诊学杂志,2007,7(5):1047-1048.
[33] Legrand VM,Serruys PW,Unger F,et al.Three-year outcome after coronary stenting versus bypass surgery for the treatment of multivessel disease. Circulation. 2004;109(9): 1114-1120.
[34] 高璀乡,李玉华,王树树,等.血管紧张素系统基因多态性与经皮冠状动脉腔内成形术加支架置入术后支架内再狭窄的关系[J].中国组织工程研究与临床康复,2007,11(43):8665-8668.
[35] 杨明,曾勇,方全,等.药物洗脱支架治疗大动脉炎致冠状动脉狭窄一例[J].中国循环杂志,2009,24(3):205-205.
[36] 焦荣华,胡名松.大鼠移植心脏组织中血小板衍生生长因子A mRNA表达及雷帕霉素的干预效应[J].中国组织工程研究与临床康复,2007,11(21):4070-4073.
[37] 张彦,杨永健.糖尿病患者接受雷帕霉素洗脱支架植入术后血管再狭窄的研究进展[J].西南军医,2014,16(2):163-165.
[38] 张立新,马长生,张海滨,等.国产雷帕霉素药物洗脱支架在冠状动脉分叉病变介入治疗中的应用[J].中国心血管病研究杂志,2007, 5(2):121-123.
[39] 张明德,曹应江,卜建学,等.急性心肌梗死置入药物洗脱支架与裸金属支架的安全性及即时效应比较[J].中国组织工程研究与临床康复,2009,13(22):4249-4252.
[40] 田锐,吕树铮,柳弘,等.药物洗脱性支架和裸金属支架植入治疗冠状动脉狭窄的临床随访观察[J].疑难病杂志,2013,12(2):90-92. |